|
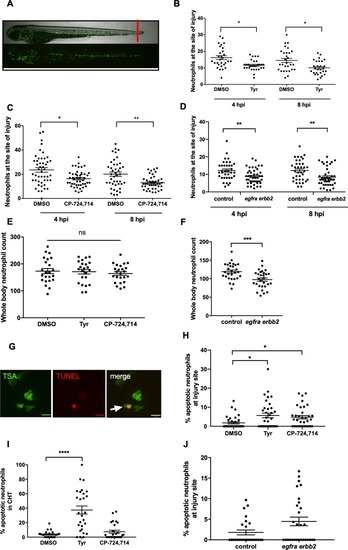
Pharmacological inhibition and genetic knockdown of <italic>egfra</italic> and <italic>erbb2</italic> by CRISPR/Cas9 reduces neutrophil number at the site of injury in a zebrafish model of inflammation.Tail fin transection was performed as indicated by the red line (A, upper image). Zebrafish larvae (mpx:GFP) were pre-treated at two dpf with DMSO, tyrphostin AG825 [Tyr, 10 µM] (B, minimum n = 28 larvae per condition), or CP-724714 [10 µM] (C, minimum n = 42 larvae per condition) for 16 hr followed by injury. egfra and erbb2 crispants were generated and injured at two dpf (D, minimum n = 36 larvae per condition). The number of neutrophils at the site of injury was determined at 4 and 8 hpi by counting GFP-positive neutrophils. To enumerate neutrophils across the whole body, uninjured inhibitor treated larvae (three dpf) (E, minimum n = 23 larvae per condition) or crispants (two dpf) (F, minimum n = 28 larvae per condition) were imaged by fluorescent microscopy (A, lower image). Apoptosis was measured at the site of injury after 8 hr by TSA and TUNEL double staining (G) (white arrow indicates TUNEL positive neutrophil, scale bar 10 μM) of mpx:GFP tyrphostin AG825 [Tyr, 10 µM] or CP-724714 [10 µM] treated larvae at three dpf (H, minimum n = 35 larvae per condition). Uninjured inhibitor treated larvae were assessed for neutrophil apoptosis in the CHT at three dpf (I, minimum n = 27 larvae per group). Apoptosis at the tail fin injury site of egfra erbb2 crispants at two dpf was also measured at eight hpi (J, minimum n = 26 larvae per group). All data collated from at least three independent experiments, displayed as mean ± SEM. Each icon shows one data point from one individual larvae. Statistically significant differences were calculated by two-way ANOVA with Sidak post-test (B–D) or one-way ANOVA with Dunnett’s post-test(E), Students’ t-test (F), Kruskal-Wallis test with Dunn’s post-test (H–I) or Mann-Whitney U test (J), and indicated as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
|