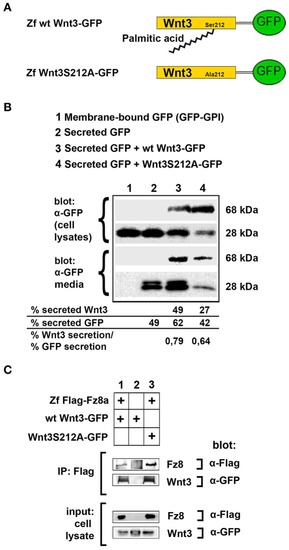
Acylation of Wnt3 at the conserved serine residue (S212) is not necessary for its secretion and interaction with its receptor. (A) Domain structure of C-terminally GFP-tagged wild-type (Zf wt Wnt3-GFP) and mutant (zf Wnt3S121A-GFP) zebrafish Wnt3. Palmitic acid is attached to the conserved serine residue. Palmitoylation is supposed not to occur in the mutant construct where serine is replaced by alanine. (B) Secretion assay for wt and mutant Wnt3 proteins and western blot of input (cell lysates) and collected media. Both wt Wnt3-GFP and Wnt3S121A-GFP are detected in the media collected from HEK293T cells transfected with corresponding constructs. Secreted and total produced levels of the proteins were calculated and used for comparisons. Membrane-bound GFP (GFP:GPI) and secreted GFP are used as negative (non-secretory protein) and positive (secretory protein) controls, respectively. Forty-nine, sixty-two, and forty-two percent of total GFP were secreted from cells transfected with secreted (sec) GFP, sec GFP+ wt Wnt3-GFP and sec GFP+ Wnt3S212A-GFP, respectively. Forty-nine and twenty-seven percent of Wnt3 were secreted from cells transfected with sec GFP+ wt Wnt3-GFP and sec GFP+ Wnt3S212A-GFP, respectively. Ratios of secreted Wnt3 to secreted GFP were 0.79 and 0.64 for wt Wnt and Wnt3S212A, respectively. Percentages represent mean of three independent experiments. (C) Coimmunoprecipitation of wt and mutant Wnt3 proteins with Frizzled8 receptor. Both wt Wnt3-GFP and Wnt3S121A-GFP coIP with Zf FLAG-Fz8a in HEK293T cells. wt Wnt3-GFP does not bind to Dynabeads non-specifically. Three independent experiments were performed.
|

