Figure 1
- ID
- ZDB-FIG-190723-1206
- Publication
- Yien et al., 2015 - Mitochondrial transport of protoporphyrinogen IX in erythroid cells
- Other Figures
- All Figure Page
- Back to All Figure Page
|
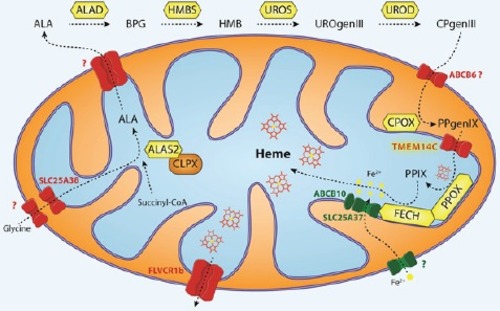
Glycine is imported via SLC25A38 and condenses with succinyl-CoA to form δ-aminolevulinic acid (ALA) in a reaction catalyzed by ALA synthase (ALAS2 in red cells) [ |