Fig. 2
- ID
- ZDB-FIG-171110-37
- Publication
- Sánchez et al., 2016 - Mechanosensory organ regeneration in zebrafish depends on a population of multipotent progenitor cells kept latent by Schwann cells
- Other Figures
- All Figure Page
- Back to All Figure Page
|
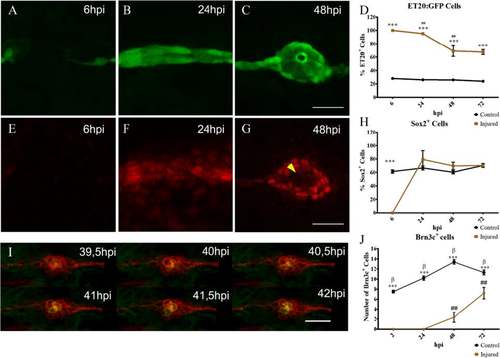
Neuromast regeneration depends on interneuromastic cell accumulation. The L3 neuromasts of 3 days post fertilization tg(et20:GFP) larvae were electroablated or left uninjured as controls, and fixed at different time points after damage (hours post injury, hpi). a–c Detection of ET20:GFP-labeled cells after electroablation. d Quantification of GFP-labeled cells at the L3 position (n = 10). Initially, in electroablated fish, all accumulating cells expressed GFP but the percentage of GFP versus total cells diminished significantly between 24 and 48 hpi (## p < 0.01). At all stages after injury, L3 neuromasts of electroablated larvae had a much higher proportion of ET20:GFP cells in comparison with control larvae (***p < 0.001). e–h Immunodetection and quantification of Sox2-expressing cells (n = 10). At 6 hpi, few, if any, Sox2-expressing cells were seen in the injury zone but, after 24 hpi, the number of Sox2-expressing cells was approximately the same as in controls (h) (***p < 0.001) . Note the loss of Sox2 expression in the most centrally located cells at 48 hpi (g, yellow arrowhead). i Images extracted from a time-lapse sequence of a double tg(cxcr4b:mCherry;brn3c:GFP) electroablated larva. The sequence reveals the progressive appearance of GFP expression in centrally located hair cells. j In vivo quantification of the number of hair cells in control and injured larvae that regenerated their neuromasts at 2, 24, 48, and 72 hpi (n = 15); ## and β indicate statistical differences within the same group, control or injured, comparing neighboring values (β p < 0.001, ## p < 0.01), while asterisks reflect statistical difference between control and injured at the same time points (***p < 0.001). Note that the ET20:GFP and Sox2 expression data corresponding to 6 and 24 hpi (shown in d and h, respectively), come from a mix of larvae committed and not committed to regenerate. This is because the samples had to be fixed at stages in which we could not distinguish between the outcomes. Scale bar a–g, i: 50 μm. Further details on replicates are provided in “Quantifications and statistical analysis” in the “Methods” section |