The Zebrafish Science Monitor Vol 3(5)
PRODUCTION OF ANDROGENETIC HAPLOIDS AND DIPLOIDSBy G.E. Corley-Smith, C.J. Lim, and B.P. Brandhorst, Institute of Molecular Biology & Biochemistry, Simon Fraser University, Burnaby, BC, V5A 1S6, CANADA
We have visual and molecular marker evidence that we have produced haploid and diploid androgenetic zebrafish. Androgenotes inherit all of their chromosomes from their father and none from their mother.
Androgenesis is a technique that can be useful in considering a variety of genetic phenomena, including: rates of meiotic recombination in spermatogenesis, detection and mapping of male specific DNA markers or linkage groups (if any exist), basis of sex determination in zebrafish, and the role of imprinting of essential paternal genes, if any.
Androgenetic haploids are produced by irradiating eggs to destroy the maternal genome, followed by fertilization. By inhibition of either karyokinesis or cytokinesis of the first mitotic division, diploid androgenotes can be produced. For irradiation of eggs we use a cabinet x-ray source that is commercially manufactured for detection of fractures in airplane and other critical parts, the Torrex 150D x-ray inspection system (Faxitron X-Ray Corp., Buffalo Grove, IL, USA; phone (708) 465-9729). The initial cost is approximately $17,000 US. When switched off, the source is extremely safe. Radioisotope sources are also useful for irradiating eggs. Disadvantages of using a radioisotope source for irradiation include: set up expenses, regulations and precautions necessary for isotope use, and the requirement of a room dedicated for irradiation purposes. X-ray dosimetry was performed with a MDH1515 dosimeter using a MDH model 10X5-180 ion chamber (paddle chamber). This was calibrated with a known 137Cs source (NBS 137Cs source #47455).
We heat shock zygotes to inhibit the first mitotic division. After fertilization, eggs are held at 28.5+/-0.5C. Eggs are heat shocked 13 minutes after fertilization for 2 minutes at 41.4+/-0.05C. Temperatures were measured with a calibrated thermometer (Fisher Scientific, Cat. No. 15041A) with an uncertainty in temperature certified not to exceed 0.03C.
In a recent experiment, we collected eggs from one female (from a line which originated from zebrafish bought from pet stores in Vancouver Island, B.C. and assumed not to be closely related to Eugene's AB line) and the milt from one male (*AB line from Charline Walker, which has been screened to reduce recessive lethals). The eggs were held in ovarian fluid at room temperature for 50 minutes, the time required for irradiation of eggs. The milt was collected just prior to being used for fertilization and was held in sperm extender. 204 eggs were irradiated with 10,000 R of x-rays. These eggs and 76 eggs which were not irradiated (control group) were then fertilized. 72% of the control group were observed developing normally at 24 hr. All of these subsequently hatched and appeared to be diploids. Of the 204 eggs that were irradiated and subsequently fertilized, 49 were not heat shocked (treatment to produce haploid androgenotes) and 155 were heat shocked to produce diploid androgenotes.
In the group whose eggs were irradiated (to destroy the maternal genome) but not heat shocked, 5 embryos developed, all of which exhibited the haploid syndrome (shortened body, small melanocytes). These are putative androgenetic haploids. In the group whose eggs were irradiated and then heat shocked to inhibit the first mitotic division, 2 normal looking diploids developed (putative diploid androgenotes).
The haploid syndrome can be seen at 24 hours as a shortened body phenotype (Figure 1). At 48 hours, the shortened body is easily noticeable and the difference in size of melanocyte starts to become noticeable (Figure 1) and is pronounced by 96 hours (not shown). The development of putative androgenetic diploid embryos was initially slightly retarded (Figure 1). However, by the end of the first month, these fish achieve approximately the same size as the diploid control fish.
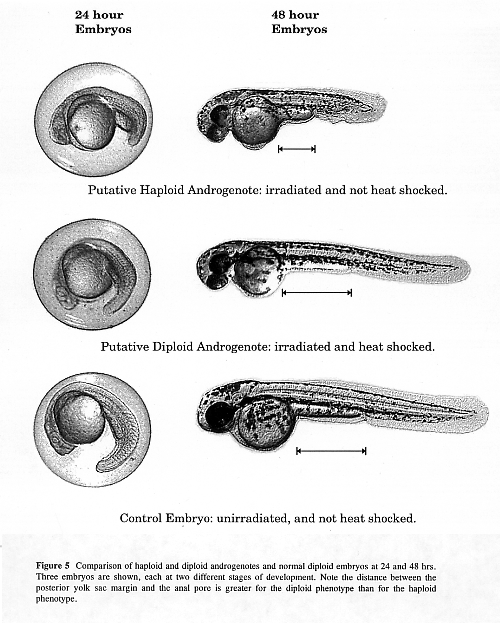
The lack of any embryos that appeared to be diploid in our irradiated and non-heat shocked group suggests that the irradiation dosage was sufficient to destroy the maternal genome, or somehow impeded its transfer to the offspring. Thus, we believe the surviving diploid embryos in the irradiated and heat shocked group, are indeed androgenetic rather than resulting from a failure to eliminate the maternal genome. Putative haploid androgenotes often hatch, but seldom feed. They usually die during the first few weeks of life. We have apparently healthy, active putative diploid androgenotes which are over a month old.
The percentage of haploid and diploid androgenotes produced relative to our control group was 14% and 2%, respectively. We believe we can increase these production rates with further refinement of the technique.
Genetic analysis was performed using polymorphic DNA markers that were fluorescently labeled during PCR and detected on an ABI 373 Automated Sequencer, thus enabling precise sizing and clear identification of markers. None of the maternal specific markers we have identified to date, some of which are homozygous, are inherited by either the haploid or diploid putative androgenotes we have produced. This does not preclude the possibility of some leakage of maternal DNA to the putative androgenotes, but it is compelling evidence that we have produced zebrafish which are lacking a substantial proportion of the maternal genome. We have confidence in our makers as all DNA markers identified in the parents, many of which are not polymorphic, were observed in at least some of the normal diploid progeny. Markers were considered homozygous if they were observed in all 12 normal diploid progeny analyzed.
The sex ratios we will observe in the progeny of our androgenetic zebrafish, may be informative about the mode of sex determination in zebrafish, which is presently not understood. The survival of diploid zebrafish androgenotes suggests a lack of male specific imprinting of essential genes as has been observed in mammalian species.
Zebrafish Science Monitor Vol 3(5)
Return to Contents
