
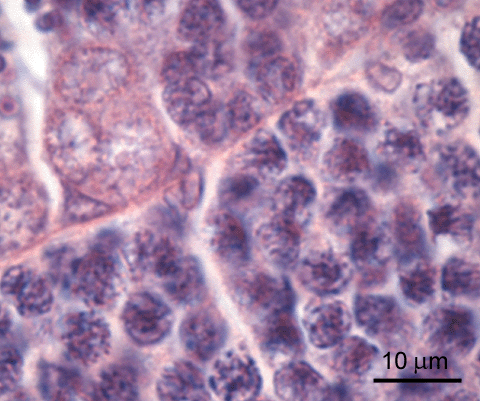
adult zebrafish; drawings and H&E staining structure of the mature testis - details
The seminiferous tubules
 are separated by thin strands of interstitial connective tissue
are separated by thin strands of interstitial connective tissue . This interstitial tissue contains connective tissue cells
. This interstitial tissue contains connective tissue cells and Leydig cells
and Leydig cells (intermediate sized cells with a round-oval nucleus), which produce androgen when stimulated by the pituitary-derived gonadotropic hormone.
(intermediate sized cells with a round-oval nucleus), which produce androgen when stimulated by the pituitary-derived gonadotropic hormone.
At the luminal side of the basement membrane which bounds the tubules, large solitary spermatogonium A cells
 can be discerned. These germ cells are a continuous source of spermatogenic cells for the actively renewing tissue. After division, these spermatogonia give rise to a generation of spermatogonia B
can be discerned. These germ cells are a continuous source of spermatogenic cells for the actively renewing tissue. After division, these spermatogonia give rise to a generation of spermatogonia B , which occur in clustes of four or more cells. After a limited number of proliferative cycles, spermatogonia B enter meiosis to give rise to spermatocytes, which are contained in the spermatocysts
, which occur in clustes of four or more cells. After a limited number of proliferative cycles, spermatogonia B enter meiosis to give rise to spermatocytes, which are contained in the spermatocysts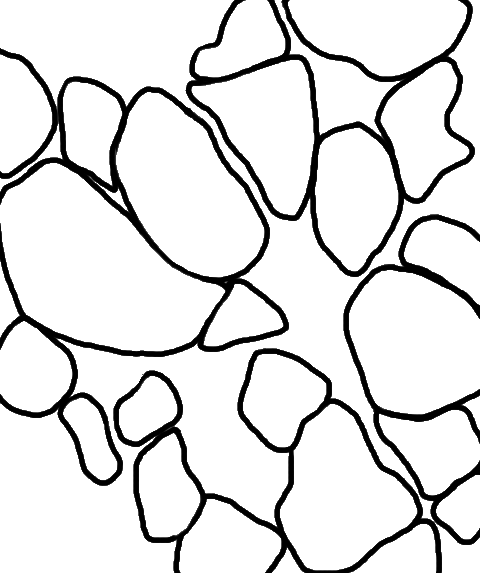 . This process and the succesive stages of spermatogenesis are illustrated in more detail in the spermatogenesis page.
. This process and the succesive stages of spermatogenesis are illustrated in more detail in the spermatogenesis page.
Proliferation of spermatogonia is induced when these cells are being enveloped by Sertoli cells
 . Sertoli cells have an irregular nucleus, and are occasionally visible at the basement membrane (see also: Sertoli cell section. They serve as a mediator in the androgenic stimulation of spermatogenic cells1.
. Sertoli cells have an irregular nucleus, and are occasionally visible at the basement membrane (see also: Sertoli cell section. They serve as a mediator in the androgenic stimulation of spermatogenic cells1.
Each cluster of meiotic cells that arises from spermatogonial division shows synchronous maturation, and such spermatocysts
 are enveloped by the thin layer of the membrane of lobule boundary cells
are enveloped by the thin layer of the membrane of lobule boundary cells , which are adapted Sertoli cells.
, which are adapted Sertoli cells.
When spermatogenesis is completed, these lobule boundary cells open up to void the mature sperm into the tubular lumen.
Spermatogonial cells occur randomly over the entire stretch of the tubules, and consequently, spermatocysts with various stages of maturation can be found anywhere. This organisation is referred to as "unrestricted", in contrast to the resticted testis type, where the spermatogonia reside at the periphery of the organ, directly under the covering tunica albuginea. These residual spermatogonia produce synchronously maturing cohorts of spermatocysts, which migrate simultaneously towards a central tubule or duct2,3.
References
References
- Nagahama-Y. Endocrine regulation of gametogenesis in fish. Int.J.Dev.Biol.38:217-229;1994.
- Grier-HJ, Linton-JR, Leatherland-JF, and De Vlaming-VL. Structural evidence for two different testicular types in teleost fishes. Am.J.Anat.159:331-345;1980.
- Loir-M, Sourdaine-P, Mendis-Handagama-SM, and Jegou-B. Cell-cell interactions in the testis of teleosts and elasmobranchs. Microsc.Res.Tech.32:533-552;1995.
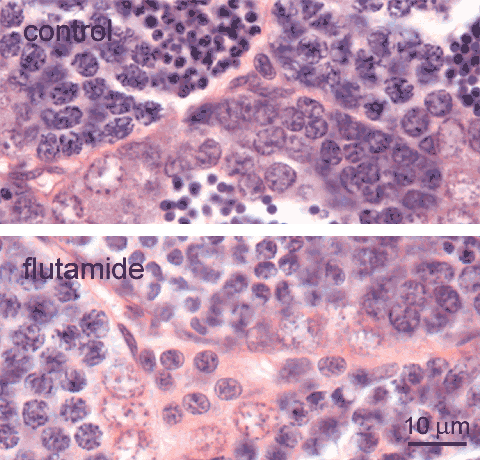
adult zebrafish, control (top), and exposed to 1 mg flutamide /L (bottom); H&E staining Leydig cells
Leydig cells
 , also known as interstital cells, are found in the interstitial space between the testis tubules. These cells have regularly shaped round or oval nuclei. In the control testis, they occur solitairy or in small clusters; larger clusters are observed after exposure to the anti-androgen flutamide, shown here [lower image] for comparison. Occasionally, such larger clusters may also be present at the periphery of the control testis. Note, that apart from Leydig cells, other cells
, also known as interstital cells, are found in the interstitial space between the testis tubules. These cells have regularly shaped round or oval nuclei. In the control testis, they occur solitairy or in small clusters; larger clusters are observed after exposure to the anti-androgen flutamide, shown here [lower image] for comparison. Occasionally, such larger clusters may also be present at the periphery of the control testis. Note, that apart from Leydig cells, other cells , such as fibroblasts and smooth muscle cells, are also present in the interstitium.
, such as fibroblasts and smooth muscle cells, are also present in the interstitium.
Leydig cells are the source of androgens. In teleosts, they produce 11-ketotestosterone under complex regulation. Androgen production in teleosts is stimulated by the pituitary derived gonadotropin, and gonadotropin production is partly under feedback control of plasma androgen levels1; furthermore, there is possibly also auto-regulation of androgen expression at the level of the Leydig cells2. The observed hyperplasia of Leydig cells after exposure to flutamide is probably a consequence of disruption of this control.
Other testis cells are: spermatogonia A
 , spermatogonia B
, spermatogonia B , spermatocytes in leptotene
, spermatocytes in leptotene , early zygotene
, early zygotene , and pachytene
, and pachytene stages; spermatids
stages; spermatids ; Sertoli cells
; Sertoli cells .
.
References
- Nagahama-Y. Endocrine regulation of gametogenesis in fish. Int.J.Dev.Biol.38:217-229;1994.
- Schulz-RW, Vischer-HF, Cavaco-JE, Santos-EM, Tyler-CR, Goos-HJ, and Bogerd-J. Gonadotropins, their receptors, and the regulation of testicular functions in fish. Comp Biochem. Physiol B Biochem. Mol. Biol. 129: 407-417; 2001.
adult zebrafish; H&E staining Sertoli cells
Sertoli cells
 are characterized by their irregular shaped nuclei. These cells are found at the luminal side of the basement membrane of the spermatogenic tubules. They occur either solitairy, or in close association with the spermatogenic cysts, which they enclose; in this condition they are also known as lobule boundary cells.
are characterized by their irregular shaped nuclei. These cells are found at the luminal side of the basement membrane of the spermatogenic tubules. They occur either solitairy, or in close association with the spermatogenic cysts, which they enclose; in this condition they are also known as lobule boundary cells.

adult zebrafish; DAB immunostaining, hematoxylin counterstaining testis - Estrogen receptors
Luciferase protein expression in testis of adult of adult wild-type (wt) or transgenic (tg) zebrafish exposed for 48 h to 1000 nM E2. In the transgenic zebrafish, an estrogen binding sequence linked to a TATA box and luciferase reporter gene was stably introduced.
Binding of a substance to endogenous estrogen receptors and the subsequent transactivation of the estrogen responsive elements result in luciferase gene induction. This system thus is indicative for the presence of estrogen receptors.
This immunohistochemical staining of paraffin-embedded sections using polyclonal anti-luciferase antibody shows expression of luciferase localized in clusters of spermatogonia
 (brown staining in tg, spermatogonia in wt are blank). From the spermatocyte stage on
(brown staining in tg, spermatogonia in wt are blank). From the spermatocyte stage on , germ cells are unstained, as are all cell types in the interstitium
, germ cells are unstained, as are all cell types in the interstitium . Wild-type nontransgenic testis is completely negative.
. Wild-type nontransgenic testis is completely negative.
Note that Leydig cells are discernible in the interstitium, as are Sertoli cells
are discernible in the interstitium, as are Sertoli cells that bound the spermatocysts.
that bound the spermatocysts.
Study by Juliette Legler1.
Reference
 are discernible in the interstitium, as are Sertoli cells
are discernible in the interstitium, as are Sertoli cells that bound the spermatocysts.
that bound the spermatocysts.Study by Juliette Legler1.
Reference
- Legler J, Broekhof JLM, Brouwer A, Lanser PH, Murk AJ, Van der Saag PT, Vethaak AD, Wester P, Zivkovic D, and Van der Burg B. A novel in vivo bioassay for (xeno-)estrogens using transgenic zebrafish. Environmental Science and Technology 34: 4439-4444; 2000.