- Title
-
RPTOR blockade suppresses brain metastases of NSCLC by interfering the ceramide metabolism via hijacking YY1 binding
- Authors
- Lin, Y., Wu, Y., Zhang, Q., Tu, X., Chen, S., Pan, J., Xu, N., Lin, M., She, P., Niu, G., Chen, Y., Li, H.
- Source
- Full text @ J. Exp. Clin. Cancer Res.
|
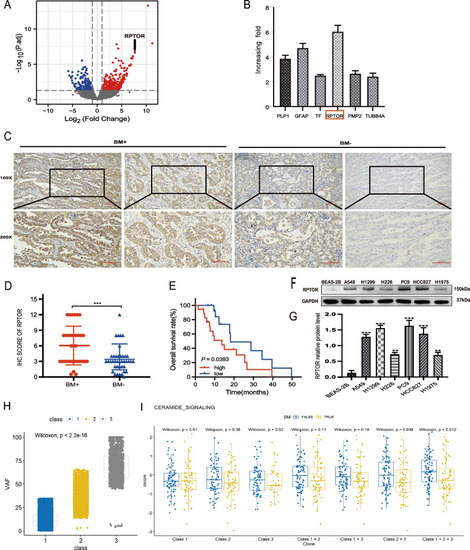
Association of high RPTOR expression or the ceramide pathway with NSCLC-BM. |
|
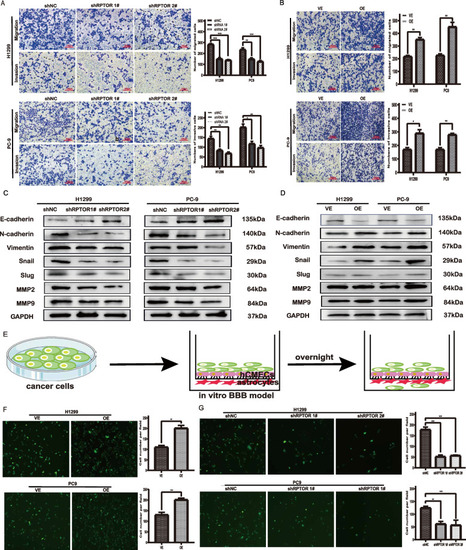
The RPTOR-promoted traverse of lung cancer cells to the BBB. |
|
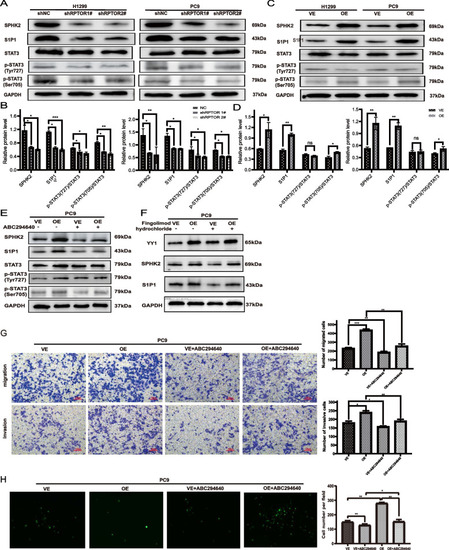
The RPTOR-promoted BM of NSCLC through the SPHK2/S1P/STAT3 signaling pathway. |
|
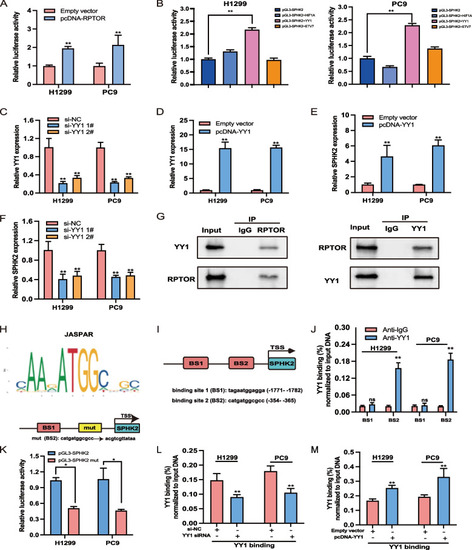
The RPTOR-regulated SPHK2/S1P pathway via binding to transcription factor YY1. |
|
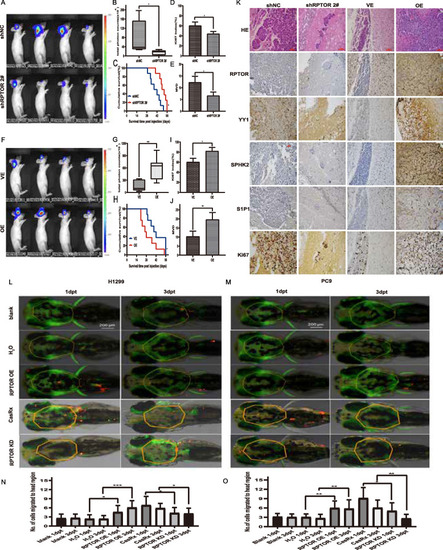
The suppressed BM of NSCLC and attenuated SPHK2/S1P/STAT3 pathway in xenograft and zebrafish model by RPTOR blockade. |
|
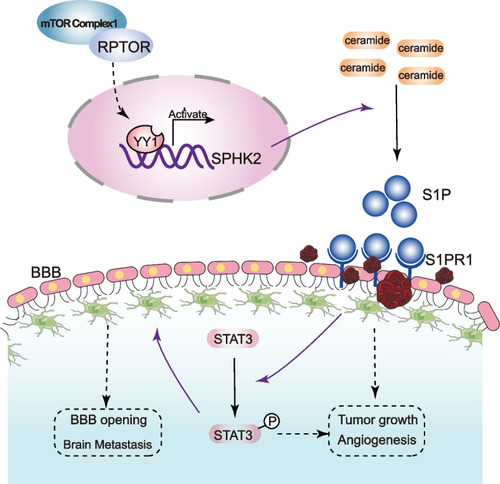
The mechanism underlying the RPTOR-promoted NSCLC-BM via the SPHK2/S1P/STAT3 signaling pathway. |