- Title
-
Smuggling on the Nanoscale-Fusogenic Liposomes Enable Efficient RNA-Transfer with Negligible Immune Response In Vitro and In Vivo
- Authors
- Hoffmann, M., Gerlach, S., Takamiya, M., Tarazi, S., Hersch, N., Csiszár, A., Springer, R., Dreissen, G., Scharr, H., Rastegar, S., Beil, T., Strähle, U., Merkel, R., Hoffmann, B.
- Source
- Full text @ Pharmaceutics
|
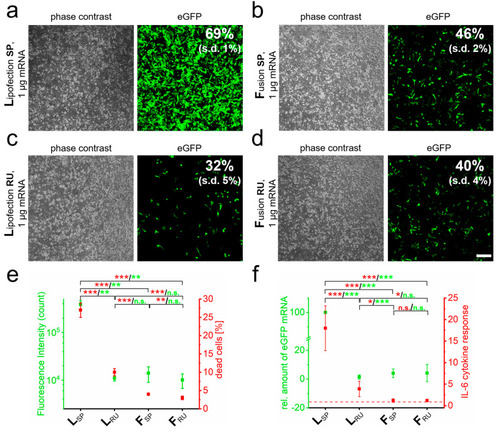
RNA quantity and transfer method influence biocompatibility and immune response. eGFP-mRNA was transferred into PC-12 cells by lipofection (L) (a) and fusion (F) (b) based standard protocols (SP) and analyzed 24 h after transfer by phase contrast and fluorescence microscopy. Transfection efficiency was determined by flow cytometry. Identical analyses with reduced uptake (RU) conditions were performed for lipofection (c) and fusion (d). Analyses of cells as indicated in a to d for cell death and fluorescence intensity are shown in (e). Cells were additionally harvested for mRNA isolation and characterized for IL-6 and eGFP by qRT-PCR (f). eGFP-mRNA was used in a concentration of 4 µg/mL for fusion and 2 µg/mL for lipofection. In all cases, a total of 1 µg was transferred per substrate. n = At least three independent experiments were used for each analysis. p-values: not significant (n.s.): p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001. |
|
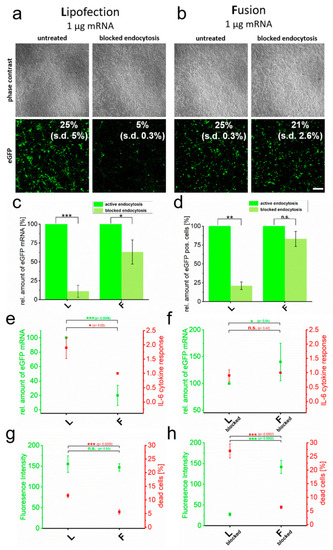
Cytotoxicity induced by RNA transfer is dependent on endocytic uptake. eGFP encoding mRNA was transferred into primary cortical neurons using endocytosis-dependent (lipofection, (a)) and -independent (fusion, (b)) transfection reagents. Transfer was performed in the absence and presence of endosome inhibitor β-MCD and cells were analyzed 24 h after treatment. Transfection efficiencies are indicated. Identically treated cells were analyzed for eGFP intensity by flow cytometry (c), relative amount of transferred eGFP mRNA (normalized to active endocytotic cells (c,d) and to the respective highest values of each measurement in (e,f)) (d), IL-6 expression (e,f) and number of dead cells (g,h). For better comparison, IL-6 values are directly compared to transferred eGFP mRNA levels (e,d). Percentage of dead cells is given in comparison to fluorescence intensity (g,h). L = lipofection, F = fusion. eGFP-mRNA was used in a concentration of 4 µg/mL for fusion and 2 µg/mL for lipofection. In all cases, a total of 1 µg was transferred per substrate. Scale bar: 200 µm, n = three independent experiments for each analysis. p-values: not significant (n.s.): p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001. |
|
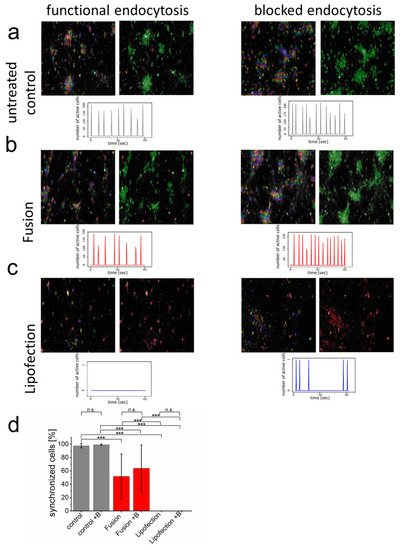
Endocytosis of nucleic acids impairs inter-neuronal network communication. (a–c) After network formation, primary cortical neurons were analyzed for functional and synchronized Ca2+ signaling (left = all identified cells, right = synchronized cells in green and inactive cells in red) in the absence and presence of endosome inhibitor β-MCD. Inhibitor was added to the culture medium two hours before cells were transfected with GFP-mRNA by endosomal-independent ((b), fusion) or endosomal-dependent ((c), lipofection) transfer mechanisms and compared to the untransfected control (a). Statistical evaluation of all experiments including s.d. (d) Here, +B: addition of endosomal inhibitor β-MCD. eGFP-mRNA was used in a concentration of 4 µg/mL for fusion and 2 µg/mL for lipofection. In all cases, a total of 1 µg was transferred per substrate. Scale bar = 200 µm. n = three independent experiments with at least 9 independently formed neuronal networks. p-values: not significant (n.s.): p > 0.05, *: p ≤ 0.05, ***: p ≤ 0.001. |
|
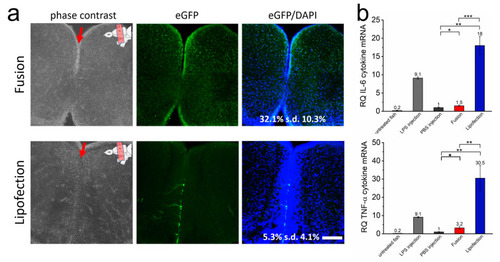
Endosome-independent transfer allows efficient transfection rates with low cytokine response in vivo. (a) The brain of adult zebrafish was injected with GFP-mRNA containing fusogenic liposomes (top) or dependent on endosomal uptake (lipofection, bottom). Injection into the ventricle is indicated by a red arrow, upper right of the image shows ventricular zone of zebrafish brain. A total of 24 h after nucleic acid transfer, whole brains were isolated and brain sections were stained for GFP (green) and nuclei (blue). Co-stained nuclei were counted as transfected cells and are given as percentage of all cells. The whole RNA of identically treated brains was isolated 24 h after nucleic acid transfer and tested for IL-6 and TNF-α expression (b). LPS was used as positive control, PBS as negative control. eGFP-mRNA was used in a concentration of 0.3 ng/µL (corresponds to 1.5 ng per injection). Scale bar = 200 µm. Each condition was analyzed on at least 5 independent injections. p-values: not significant (n.s.): p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001. |
|
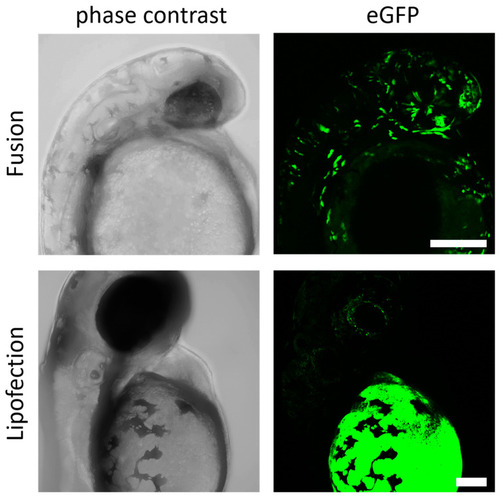
Fusogenic liposomes mediate efficient expression in zebrafish embryos. GFP-mRNA was injected into the zebrafish yolk at 4.5 hpf by endosome-independent (fusion) and endosome-dependent (lipofection) mechanisms. Embryos were analyzed for GFP expression 24 h (fusion) and 48 h (lipofection) after injection. eGFP-mRNA was used in a concentration of 0.3 ng/µL (corresponds to 1.5 ng per injection). Scale bar = 200 µm. |