- Title
-
A Missense Variant in PDK1 Associated with Severe Neurodevelopmental Delay and Epilepsy
- Authors
- Vaz, R., Wincent, J., Elfissi, N., Rosengren Forsblad, K., Pettersson, M., Naess, K., Wedell, A., Wredenberg, A., Lindstrand, A., Ygberg, S.
- Source
- Full text @ Biomedicines

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
(A) Genome analysis revealed a de novo variant in PDK1. Whole genome sequencing showed a c.1139G > A (p.G380D) variant in exon 11 of PDK1 (*) in heterozygosity (arrow). Sanger sequencing of the parents and the proband confirmed it was a de novo variant. (B) Partial amino acid sequence alignment including the amino acid affected by the variant (*) shows conservation between human and zebrafish. |
|
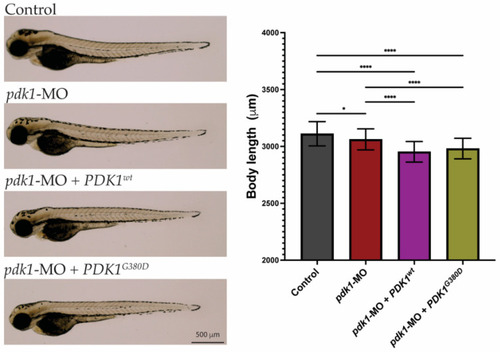
Morphology of zebrafish embryos. Three-days-old embryos from all experimental groups were photographed and show no major phenotypes. Quantification of total body length showed embryos from the experimental groups were significantly smaller than those from the control group (n = 50–75 embryos per group, * p < 0.026, **** p < 0.0001). PHENOTYPE:
|
|
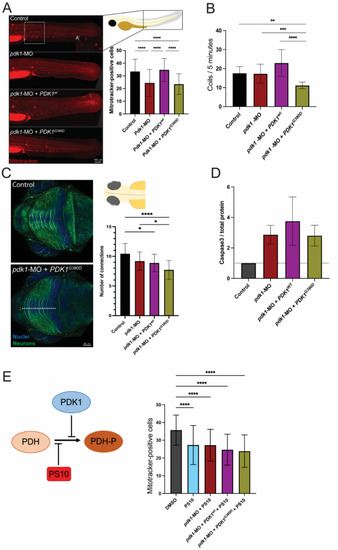
Expression of PDK1G380D in zebrafish embryos affects embryogenesis. (A) Mitochondria with membrane potential were labeled using a Mitotracker labeling assay and the quantification was performed by counting the positively stained cells (see inset for close up on positive cells) in an area of 300 × 350 µm in the trunk of the embryo (white box). (A’) Mitotracker-positive cells shown in higher magnification. A significant decrease in the number of cells with labeled mitochondria was found in pdk1-MO + PDK1G380D when compared to control and pdk1-MO + PDK1wt embryos (n = 40–45 per group, **** p < 0.0001). (B) Expression of mutant PDK1 in zebrafish embryos resulted in a significant decrease in spontaneous movements (coils) when compared to the others experimental groups (n = 108–120 embryos per group, ** p = 0.003, *** p = 0.004, **** p < 0.0001). (C) Neuronal differentiation was assessed by labelling embryos with acetylated-a-tubulin (neurons, in green) followed by quantification of the neuronal projections in the tectum (top view). We found a significant decrease in the number of projections crossing the white dotted line of embryos injected with pdk1-MO + PDK1G380D when compared to control embryos (n = 30 embryos per group, * pcontrol vs. pdk1-MO = 0.011 and pcontrol vs. pdk1-MO + PDK1wt = 0.0214, **** p < 0.0001). (D) Apoptosis was increased in experimental groups when compared to control embryos, detected by Western blot, but it does not correlate with the severity of the other phenotypes analyzed (quantification of cleaved Caspase-3 normalized to total protein, n = 4 protein extracts per group). (E) PS10, a compound known to inhibit the phosphorylation of PDH-E1α upstream of PDKs, inhibits the rescue by PDKwt, and supports the hypomorphic nature of PDK1G380D (n = 52–70 embryos per group, **** p < 0.0001). |
|
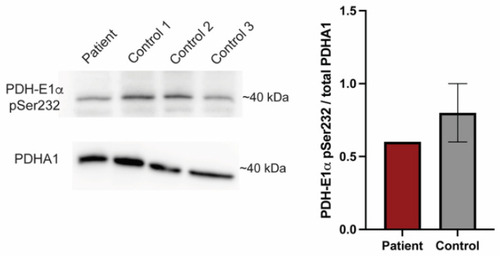
PDK1G380D causes a reduction in phosphorylated PDH-E1α. Phosphorylated PDH-E1α (PDH-E1α pSer232) is reduced in protein extracts from our patient fibroblasts when compared to three controls, which is consistent with the findings in zebrafish. The quantification was normalized to total PDHA1. |