- Title
-
Zebrafish endochondral growth zones as they relate to human bone size, shape and disease
- Authors
- Le Pabic, P., Dranow, D.B., Hoyle, D.J., Schilling, T.F.
- Source
- Full text @ Front Endocrinol (Lausanne)
|
General overview of the intramembranous and endochondral composition of the zebrafish and human skeletons. (A) Human adult skeleton. In the head, intramembranous bones such as those of the calvaria (the top portion of the neurocranium) dominate the human skull in surface area, while endochondral bones mostly occupy the cranial base. All of the bones that compose the trunk and appendicular skeletons are endochondral, except for portions of the clavicle and scapula. (B) Zebrafish adult skeleton. The zebrafish skull, trunk and appendage skeletons are composed of both intramembranous and endochondral bones. The zebrafish skull is composed of 134 bones, 78 of which are endochondral. The zebrafish trunk skeleton is composed of intramembranous vertebrae and ribs. The zebrafish appendage skeleton is composed mostly of endochondral bones, while the fin ray exoskeleton is completely intramembranous. |
|
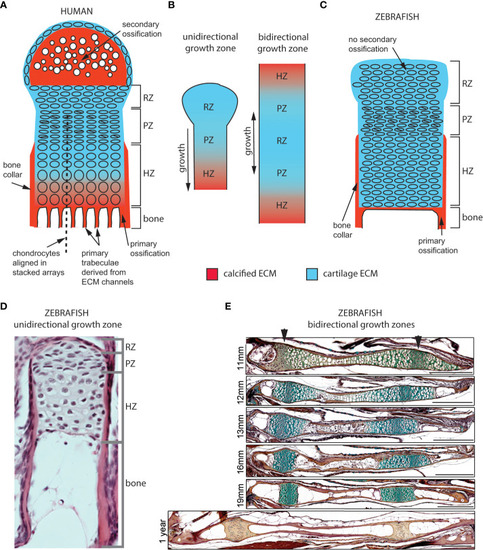
Cellular organization of epiphyseal and synchondroseal growth zones. (A) Human growth plate chondrocytes transition through resting-, proliferative- and hypertrophic zones (RZ, PZ, and HZ, respectively) before dying or transitioning to an osteoblast fate at the chondro-osseous junction. Cartilage cells stop dividing and enlarge in the hypertrophic zone. The bone collar forms a sheath around hypertrophic chondrocytes; the secondary ossification flanks the growth plate distally. Primary bone trabeculae derived from extracellular matrix channels populate the bone cavity. (B) In unidirectional (epiphyseal) growth zones, the resting zone is distal to the proliferative zone, itself distal to the hypertrophic zone; this layout produces unidirectional growth. In bidirectional (synchondroseal) growth zones, the resting zone is flanked by two proliferative zones and two hypertrophic zones in a mirror image organization; this layout produces bidirectional growth. (C) Stereotypical zebrafish unidirectional growth zone organization: chondrocytes transition through RZ, PZ and HZ, but they do not enlarge in the HZ. At the zebrafish resorption front, chondrocytes die or transition to either an osteocyte or adipocyte fate. A perichondral bone collar sheathes the zebrafish hypertrophic zone, but no secondary ossification is associated with zebrafish epiphyseal growth zones. Trabeculae are not observed in smaller teleosts such as zebrafish. (D) Histological section of zebrafish proximal radial showing unidirectional endochondral growth zone [originally published in (23)]. (E) Time series of maturation at two zebrafish bidirectional growth zones located within the ventral (left) and dorsal (right) ceratohyal synchondroses [originally published in (24)]. (scale bars = 50 µm). |
|
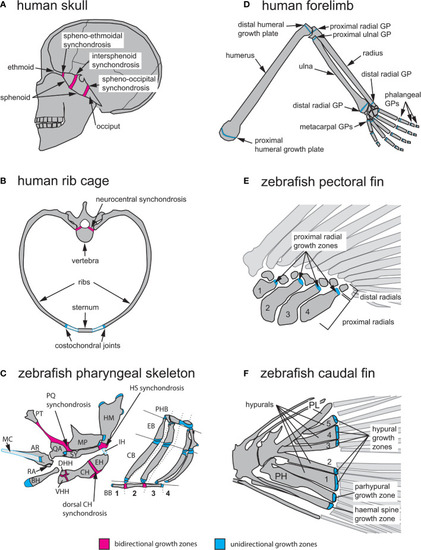
Anatomical locations of endochondral growth zones in human vs zebrafish. (A) Three synchondroses mediate growth of the human skull base (depicted in sagittal view): the spheno-ethmoidal-, intersphenoid- and spheno-occipital synchondroses. (B) Endochondral growth of human vertebrae and ribs (depicted in transverse view) takes place at neurocentral synchondroses and costochondral joints, respectively. (C) Over thirty endochondral growth zones mediate zebrafish pharyngeal skeleton growth. Three highly visible synchondroses mediate growth of first and second pharyngeal arch (PA1-2) skeletons. The PQ synchondrosis mediates growth of the QA and MP. The HS synchondrosis mediates growth of the SY and HM. The dorsal CH synchondrosis mediates growth of the CH and EH. In PA1 and 2, epiphyseal growth zones are found at the posterior MP, anterior SY, dorsal HM and anterior BH. In the gill supporting skeleton, epiphyseal growth zones are found at the ends of each CB and EB bone. (D) Axial growth of human stylopod (humerus, femur) and zeugopod (radius, ulna, tibia, fibula) bones is mediated by epiphyseal growth zones (=growth plates) located at each bone extremity. A single proximal epiphyseal growth zone mediates axial elongation of each autopod long bone (hand and foot phalanges). (E) In the zebrafish pectoral fin, epiphyseal growth zones are found at the distal end of each proximal radial. (F) In the zebrafish caudal fin, epiphyseal growth zones located at the distal end of each hypural bone (H1-5), the prehypural and two last haemal spines mediate axial elongation. AR, articular; BB, basibranchials; BH, basihyal; CB, ceratobranchial; CH, ceratohyal; DHH, dorsal hypohyal; EB, epibranchial; EH, epihyal; HM, hyomandibular; HS, hyosymplectic; IH, interhyal; MC, Meckel’s cartilage; MP, metapterygoid; PH, parhypural; PHB, pharyngobranchials; PL, pleurostyle; PT, palatine; QA, quadrate; RA, retroarticular; SY, symplectic; VHH, ventral hypohyal. Red indicates bidirectional- and blue indicates unidirectional growth zones. |
|
Early anatomy of zebrafish endochondral growth zones. (A) Endochondral growth zones start to appear in the zebrafish skeleton around 12days post-fertilization (dpf). One or more ossification centers appear on each bone. Unossified cartilage regions at bone ends become unidirectional growth zones, while those flanked by ossifying cartilage become bidirectional growth zones. In the 12dpf pharyngeal skeleton, the QA and MP bones ossify over the PQ cartilage, the HM and SY bones ossify over the HS cartilage, the HH, CH and EH bones ossify over the CH cartilage, BB1 and 2 ossify over the BB cartilage. Single ossifications appear on other pharyngeal bones. (B) In the caudal fin endoskeleton, single ossifications appear on each cartilage element, resulting in a single distal endochondral growth zone per element. (C) In the pectoral fin endoskeleton, the endoskeletal disc is progressively carved into four proximal- and seven distal radials. Ossification of each proximal radial leaves a single endochondral growth zone at the distal end. Distal radials do not ossify. BB, basibranchial; BH, basihyal; CB, ceratobranchial; CC, compound centrum; DR, distal radial; ED, endoskeletal disc; H, hypural; HB, hypobranchial; IH, interhyal; MC, Meckel’s cartilage; NO, notochord; PH, parhypural; PHB, pharyngobranchials; PR, proximal radial; SCO, scapulocoracoid, VHH, ventral hypohyal. |
|
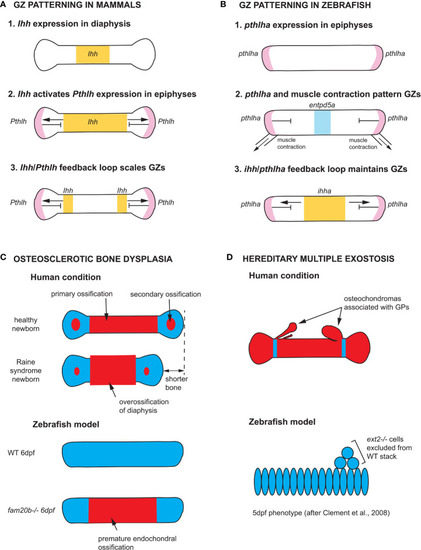
Zebrafish models of endochondral growth zone development and disease (A) Model for the patterning of growth zones (GZs) based on genetic studies of mouse long bones (82–84). Indian hedgehog (Ihh) is first expressed in the nascent diaphysis of the cartilage model. Its expression domain expands towards the epiphyses and activates Parathyroid hormone-like hormone (Pthlh) expression in periarticular cartilage. Pthlh represses Ihh at a distance, which sets the distance between the hypertrophic zone (HZ) and resting zone (RZ). (B) Model for the patterning of CH GZs in zebrafish based on (46). pthlha is expressed at the epiphyses of the differentiating CH. The HZ is then patterned by pthlha and muscle contractions before the onset of ihha expression. According to this model, ihha plays a role in the maintenance of GZs, not their patterning. (C) Zebrafish fam20b mutants recapitulates the skeletal phenotype of Raine syndrome, a particular form of osteosclerotic bone dysplasia (45). Short overossified long bones are observed in Raine syndrome newborns. Premature ossification of the CH diaphysis is observed in zebrafish fam20b mutants. (D) Zebrafish chimaeras recapitulate the formation of cartilage nodules observed in the human condition hereditary multiple exostosis, which results from a mutation in the EXT2 gene. ext2-/- chondrocytes are excluded from WT cartilage stacks in zebrafish chimaeras, leading to the hypothesis that osteochondromas observed in EXT2+/- patients result from loss-of-heterozygosity (85). Ihh and ihha expression domains in yellow, Pthlh and pthlha expression domains in pink, ectonucleoside triphosphate diphosphohydrolase 5a (entpd5a) expression domain in light blue, cartilage in blue, and bone in red. CH, ceratohyal; GPs, growth plates; WT, wild type; dpf, days post-fertilization. |