- Title
-
Zebrafish patient-derived xenograft models predict lymph node involvement and treatment outcome in non-small cell lung cancer
- Authors
- Ali, Z., Vildevall, M., Rodriguez, G.V., Tandiono, D., Vamvakaris, I., Evangelou, G., Lolas, G., Syrigos, K.N., Villanueva, A., Wick, M., Omar, S., Erkstam, A., Schueler, J., Fahlgren, A., Jensen, L.D.
- Source
- Full text @ J. Exp. Clin. Cancer Res.
|
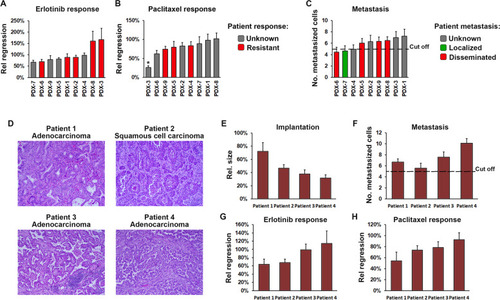
ZTX models can be established directly from patient samples and accurately predict patient treatment outcomes. |
|
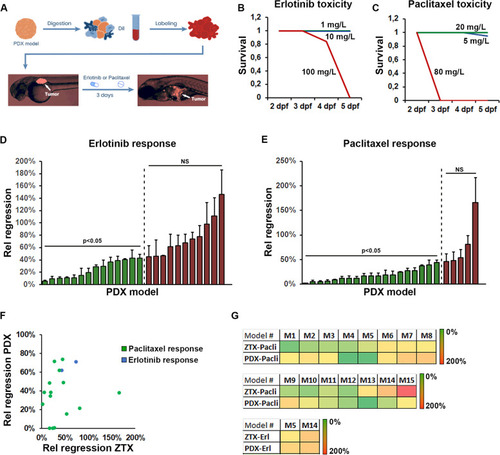
Robust implantation and growth of NSCLC PDX models in zebrafish larvae. |
|
Similar response to erlotinib and paclitaxel are seen in ZTX and mouse PDX models. |
|
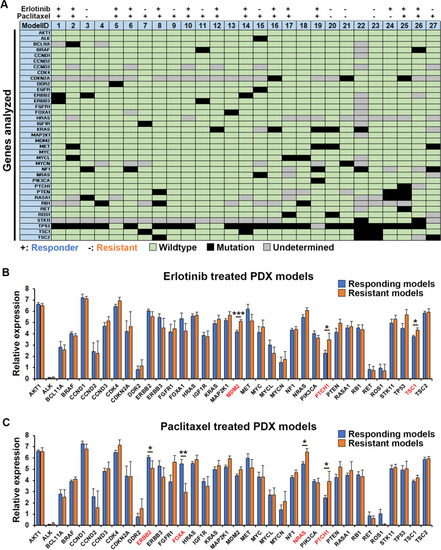
Genetics of tumors and correlation with treatment outcome. |
|
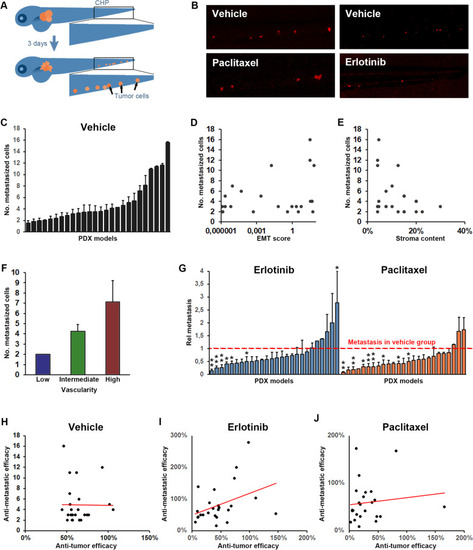
Tumor dissemination correlates with vascularity of the tumor but not tumor growth/regression rate. A Cartoon illustrating dissemination of tumor cells (shown in orange) to the caudal hematopoietic plexus (marked by the black squares) three days following tumor implantation in the perivitelline space. B Representative images of tumor cells (shown in red) in the caudal hematopoietic plexus three days after tumor implantation for a representative model in which dissemination was moderately but significantly inhibited by treatment with Erlotinib (10 mg/L, right panels) but not by treatment with Paclitaxel (20 mg/L, left panels). C Quantification of the average number of cells that at three days post implantation have disseminated to the caudal hematopoietic plexus (metastasized cells) for each of the implantable models. D-F Quantification of the EMT-score (D), stromal content (E) and vascularity (F) of the PDX models when grown in mice plotted against the average number of metastasized cells, as quantified in C, three days after implantation of the models in zebrafish larvae. Positive correlation was significant for metastasis and vascularity but not for metastasis and EMT-score or stromal content. n = 12–33 per group. G Quantification of the relative change in metastasis to the caudal hematopoietic plexus in treatment compared to vehicle groups after treatment with either Erlotinib (10 mg/L, shown in light blue), or Paclitaxel (20 mg/L, shown in orange). Red dashed line indicates the control group for each model. n = 7–22 per group. H-J Quantification of the changes in tumor size between day three and zero for larvae in the control group (H) or after treatment with Erlotinib, 10 mg/L (I), or Paclitaxel, 20 mg/L (J), divided by the changes in tumor size between day three and zero for larvae in the corresponding control groups (anti-tumor efficacy) and plotted against the relative change in metastasis as quantified in G, for each of the 25 implantable models. A non-significant trend towards a positive correlation was observed in the Erlotinib treated group but not in the Paclitaxel treated groups. n = 7–22 per group |
|
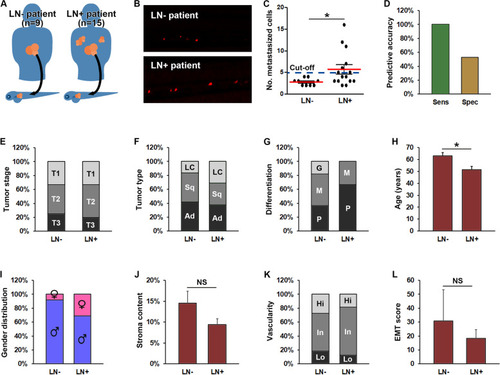
Increased metastasis in ZTX models accurately predicts lymph node involvement in the patients. A Cartoon illustrating implantation of PDX models generated from non-small cell lung cancer patients with or without lymph node involvement (LN+, right part of the figure and LN-, left part of the figure respectively) in zebrafish larvae. B Representative fluorescent micrographs of tumor cells (shown in red) disseminated to the caudal hematopoietic plexus three days after implantation from patients having lymph node negative (LN-) or positive (LN+) disease upon diagnosis. C Quantification of the average number of tumor cells disseminated to the caudal hematopoietic plexus (metastasized cells) three days following implantation of PDX models generated from patients with lymph node negative (LN-) or positive (LN+) disease. Dashed line indicates the establish diagnostic cut-off of 5 disseminated cells. *: p < 0.05. n = 12–33 larvae per model. D Quantification of the diagnostic sensitivity (accuracy of predicting patients having lymph node positive disease) and selectivity (accuracy of predicting patients not having lymph node positive disease) using the cut-off value shown in C. n = 7 and 17 for sensitivity and specificity respectively. E Distribution of tumor stages associated with lymph node negative (LN-) or positive (LN+) disease. No stage was significantly associated with lymph node involvement. The number of patients associated with each group is given in Supplemental Table 1. F-I distribution of histological tumor sub-types (F), tumor differentiation stages (G), average age (H), and gender (I), among patients with lymph node negative (LN-) or positive (LN+) disease upon diagnosis. Female gender, younger age, large cell carcinoma and poorly differentiated cancer were significantly associated with higher risk of lymph node positive disease (p < 0.05). The number of patients in each group is given in Supplemental Table 1. LC: Large cell carcinoma. Sq: Squamous cell carcinoma. Ad: Adenocarcinoma. J-L Quantification of the average stromal content (J), vascularity (K) and EMT-score (L) of PDX models grown in mice derived from patients with either lymph node negative (LN-) or positive (LN+) disease upon diagnosis. n-values for each group are given in A. NS: non-significant. G: Good. M: Moderate. P: Poor. Hi: High. In: Intermediate. Lo: Low |