- Title
-
Heme-binding protein CYB5D1 is a radial spoke component required for coordinated ciliary beating
- Authors
- Zhao, L., Xie, H., Kang, Y., Lin, Y., Liu, G., Sakato-Antoku, M., Patel-King, R.S., Wang, B., Wan, C., King, S.M., Zhao, C., Huang, K.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
Phenotypes of zebrafish cyb5d1 mutants. (A and B) Whole-mount in situ hybridization results showing cyb5d1 expression in 14 somite (14 s) and 24 hpf embryos. OV, otic vesicle; KV, Kupffer’s vesicle; FL, floor plate; PD, pronephric duct; OP, olfactory placode. (C) Diagram showing the protein and genomic structures of zebrafish Cyb5d1. The corresponding nucleic acid sequences of wild type (wt) and mutant alleles are shown at the bottom. The target sequence of the sgRNA is underlined, and the PAM sequence is indicated in red. Arrow points to the truncation site of Cyb5d1 in the mutant. (D) Phenotypes of otoliths in the OV of wt and cyb5d1 mutants at different stages as indicated. Arrowheads indicate otoliths in the OV of 24 hpf embryos. (Scale bar, 100 μm.) (E) Bar graphs showing the percentage of embryos with two (normal; N) or three otoliths (three separate, 3S; three with two fused together, 3F) in different groups of embryos as indicated. Examples of embryos with 3S or 3F otoliths are shown in SI Appendix, Fig. S1. n = 447 for cyb5d1 mutants and n = 449 for wild type larvae. (F) Kymographs showing the beating pattern of multiple cilia bundles in the olfactory pit of wild type (cMO) and cyb5d1 morphants (cyb5d1 MO) in 72 hpf. (G) Beat frequency of otic vesicle motile cilia in wild type and cybd51 mutant embryos at the 23 somite stage. (H) Beat frequency of olfactory cilia in wild type (cMO) or cyb5d1 morphants (cyb5d1 MO) at 72 hpf. n.s., not significant; ***P < 0.001. Error bars are SDs for the means. EXPRESSION / LABELING:
PHENOTYPE:
|
|
CrCYB5D1 is essential for normal motility in Chlamydomonas. (A) Schematic diagram of the genomic structure of CrCYB5D1 illustrating the location of the insertion in the Crcyb5d1 mutant. The AphVIII expression cassette is inserted into the fourth exon. (B) PCR analyses of CrCYB5D1 using genomic DNA of HS211 and Crcyb5d1 as templates, using the primers shown in A. (C) Transcriptional analysis of CrCYB5D1 in the indicated strains using RT-PCR. (D) Immunoblot of whole-cell lysates from the indicated strains using antibody against mCherry; α-tubulin served as a loading control. (E) The swimming tracks of individual cells from the indicated strains were recorded for a 5 s period using a micro image analyzer (Olympus). The red and green lines indicate the straight and curved motion tracks, respectively; dark blue dots are nonmotile cells. (Scale bars, 100 μm.) (F) Bar graph showing the percentage of forward swimming cells in the indicated strains. (G) The forward swimming velocity of cells in the indicated strains. For F and G the number of cells (n) measured is indicated. ***P < 0.001. |
|
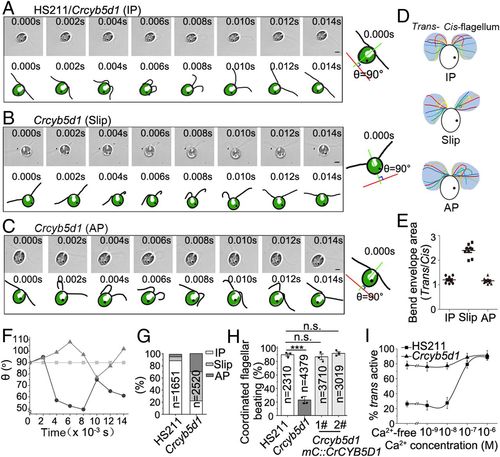
CrCYB5D1 is responsible for controlling cis–trans flagellar dominance. Real-time images and schematic diagrams of the flagellar beat waveforms in IP (HS211 or Crcyb5d1) (A), Slip (Crcyb5d1) (B), and AP (Crcyb5d1) (C) states: IP, in-phase; Slip, phase slip; AP, antiphase. The schematic diagrams at right show the principle for comparing the waveforms of cis- and trans-flagella using the rotating angle of the cell body. The green line was set between the base of the two flagella, bisects the cell body, and changed over time. The red line is perpendicular to the green line at 0.000 s and was fixed during the analysis. The angle (θ) between the red and green lines was used to monitor the rotation of the cell body and was 90° at 0.000 s. Images were taken at 500 frames per second using a high-speed video microscopy. The time of each image is indicated. (Scale bars, 5 μm.) (D) Typical sequential flagellar bending patterns in a single beat cycle were reconstructed from the waveforms in A, B, and C. (E) Dot graph showing the ratio of bend envelope area between the trans- and cis-flagellum. All values are shown as the mean ± SD. (F) Comparing the angle (θ) of each beating waveform over time in HS211 and Crcybd51: IP, Box-marked line; AP, Triangle-marked line; Slip, Round-marked line. (G) Comparison of the percentage of IP, Slip, and AP states in beat cycles of HS211 and Crcyb5d1. The number of cycles is indicated. (H) Percentage of coordinated beat cycles in the indicated strains. All values are shown as the mean ± SEM of three independent experiments. ***P < 0.001. (I) The effect of Ca2+ concentration on the dominance of cis- and trans-axonemes in HS211 and Crcybd51. Each point shows the average and the SD from three experiments. The number of cells counted in each experiment was larger than 150. |
|
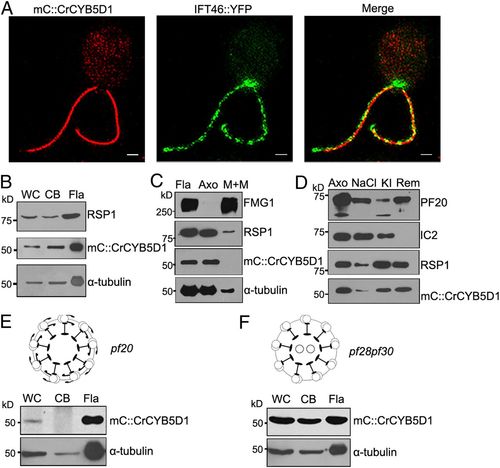
CrCYB5D1 is a radial spoke protein. (A) Localization of mC::CrCYB5D1 and IFT46::YFP in the flagella of Crcyb5d1 mC::CrCYB5D1 using 3D-SIM. (Scale bars, 2 μm.) (B) Immunoblot analysis of equal amounts of protein from whole-cells (WC), cell bodies (CB), and isolated flagella (Fla) of Crcyb5d1 mC::CrCYB5D1 probed with antibodies against RSP1, mCherry, and α-tubulin. (C) Immunoblot analysis of flagella, axonemes, and membrane plus matrix isolated from Crcyb5d1 mC::CrCYB5D1 probed with antibodies against FMG-1, RSP1, mCherry, and α-tubulin. FMG-1 is a flagellar membrane marker and RSP1 acts as a radial spoke marker. (D) Immunoblot analysis of a NaCl extract and subsequent KI extract of axonemes from Crcyb5d1 mC::CrCYB5D1 probed with antibodies against PF20, IC2, RSP1, and mCherry. PF20 was used as a central pair marker and IC2 as an outer arm dynein marker. Immunoblot analysis of equal amounts of protein from whole-cells, cell bodies, and isolated flagella of pf20 mC::CrCYB5D1 (E) and pf28pf30 mC::CrCYB5D1 (F) probed with antibodies against mCherry and α-tubulin. |
|
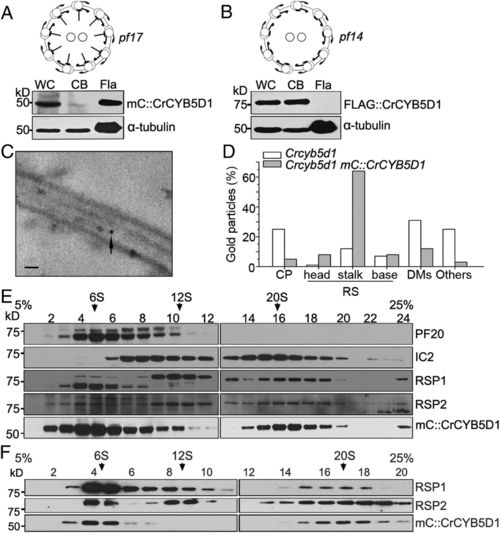
CrCYB5D1 localizes to the radial spoke stalk. Immunoblot analysis of equal amounts of protein from whole-cells, cell bodies, and isolated flagella of strains pf17 mC::CrCYB5D1 (A) and pf14 FLAG::CrCYB5D1 (B) probed with antibodies against mCherry and FLAG, respectively. (C) Representative immunogold electron micrograph of CrCYB5D1 localized at the stalk of the flagellar radial spoke using anti-mCherry antibody and a gold-conjugated secondary antibody. (Scale bar, 100 nm.) (D) The percentage of gold particles localized to different axoneme-associated components including the central pair (CP), radial spoke (RS) head, stalk and base, and the doublet microtubules (DMs). (E) Proteins from a whole-cell lysate of Crcyb5d1 mC::CrCYB5D1 were separated using sucrose density gradient centrifugation and collected in 24 fractions. Each fraction was subject to immunoblot analysis using antibodies against PF20, IC2, RSP1, RSP2, and mCherry. (F) Proteins from a flagellar membrane-matrix fraction of Crcyb5d1 mC::CrCYB5D1 were separated using sucrose density gradient centrifugation and collected in 20 fractions. Each fraction was subject to immunoblot analysis using antibodies against RSP1, RSP2, and mCherry. |
|
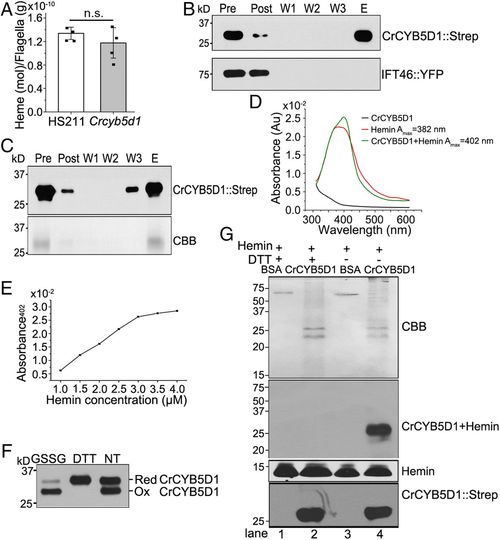
CrCYB5D1 binds heme in a redox-sensitive manner. (A) Comparison of the heme concentration in flagella of HS211 and Crcyb5d1. All values are shown as the mean ± SD of four independent experiments. n.s., not significant. Immunoblot analysis of the binding activity of CrCYB5D1::Strep from whole-cell lysate (B) or freshly purified CrCYB5D1::Strep (C) from HS211 cells expressing CrCYB5D1::Strep using hemin-conjugated agarose and an anti-Strep antibody (Upper); IFT46::YFP is a negative control in B. In C, the total protein was assessed by Coomassie blue staining (CBB, Lower). “Pre,” the soluble fraction of the cell lysate before incubation with hemin-conjugated agarose beads; “Post,” the soluble fraction after incubation with hemin-conjugated agarose beads; “W1,” “W2,” and “W3,” wash fractions; “E,” the bound fraction eluted with SDS and β-mercaptoethanol. (D) The absorbance spectra between 300 and 600 nm were collected for 3 μM CrCYB5D1::Strep, 50 μM hemin, and 50 μM hemin mixed with 3 μM CrCYB5D1::Strep. (E) The maximum absorbance (Amax) at 402 nm resulting from heme binding to CrCYB5D1::Strep was plotted for a concentration series of hemin mixed with 3 μM CrCYB5D1::Strep. (F) Assessment of the redox state of purified CrCYB5D1::Strep from whole cells. 50 mM GSSG or 1 mM DTT were used as oxidative and reductive treatments, respectively; NT indicates no treatment. The quantitative ratio of reduced and oxidized CrCYB5D1 in F under different conditions (see main text) was determined by immunoblot analysis. (G) Detection of heme-promoted peroxidase activity using purified CrCYB5D1::Strep. The top shows Coomassie blue staining; the second panel indicates peroxidase activity of the CrCYB5D1–hemin complex; the third panel shows peroxidase activity with free hemin, and the bottom shows immunoblot analysis of samples probed with Strep antibody. |
|
CrCYB5D1 participates in redox-dependent regulation of coordinated flagellar beating. (A) Assessment of the redox state of CrCYB5D1::Strep in isolated flagella treated with 1 mM H2O2 or 1 mM DTT; NT indicates no treatment. (B) Summary of identified disulfide bonds from the samples in A by PRM MS. The number of peptide spectral matches (PSMs) are indicated. (C) Comparison of heme-binding activity of CrCYB5D1::Strep from the flagella of DTT- or H2O2-treated cells using hemin-conjugated agarose;. NT indicates no treatment. “P,” the soluble fraction of the flagella before incubation with hemin-conjugated agarose beads; “E,” the bound fraction eluted with SDS and β-mercaptoethanol. (D) Immunoblot analysis of flagellar samples from HS211 and Crcyb5d1 cells treated with 1 mM DTT or 1 mM H2O2 using antibody against outer arm dynein LC3; α-tubulin served as a loading control. (E and F) Percentage of HS211 and Crcyb5d1 cells exhibiting coordinated flagellar beating upon treatment with 1 mM DTT, 40 mM TEMPOL, 0.5 mM t-BOOH, or 2 mM H2O2. The number of beat cycles is indicated. All values are shown as the mean ± SEM of three independent experiments. ***P < 0.001. n.s., not significant. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|