- Title
-
New Prenylated Indole Homodimeric and Pteridine Alkaloids from the Marine-Derived Fungus Aspergillus austroafricanus Y32-2
- Authors
- Li, P., Zhang, M., Li, H., Wang, R., Hou, H., Li, X., Liu, K., Chen, H.
- Source
- Full text @ Mar. Drugs
|
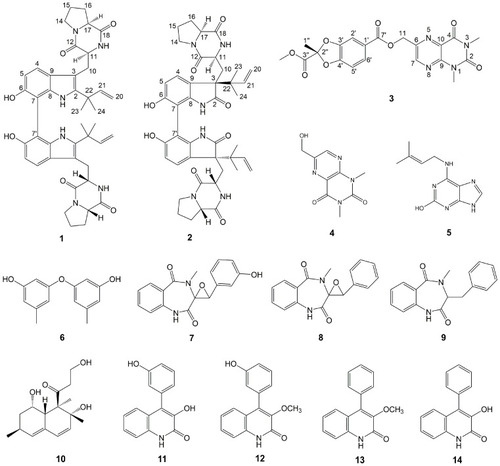
Structures of Compounds 1–14. |
|
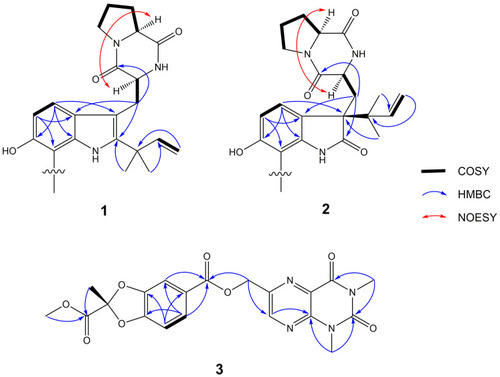
The 1H, 1H-correlation spectroscopy (1H, 1H-COSY), key heteronuclear multiple-bond correlation spectroscopy (HMBC) and nuclear overhauser effect spectroscopy (NOESY) correlations of compounds 1, 2 (only half showed) and 3. |
|
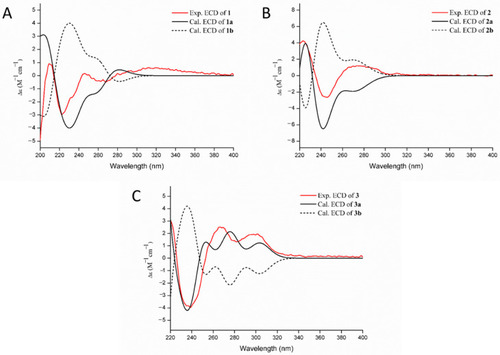
Measured CD and calculated equivalent circulating density (ECD) curves of compounds 1 (A), 2 (B) and 3 (C). |
|
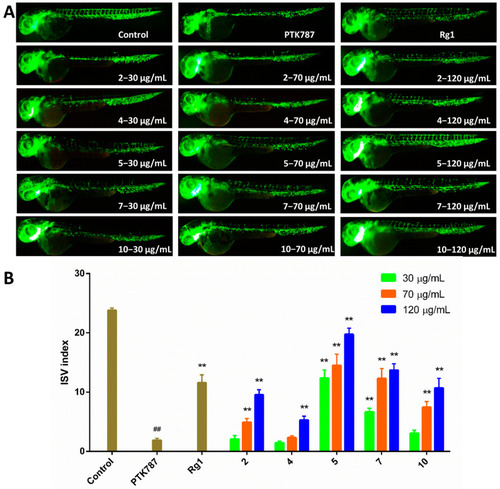
Results of pro-angiogenesis activities. (A) Typical images of intersomitic vessels (ISV) in transgenic fluorescent zebrafish (Tg (vegfr2: GFP)) treated with PTK787 and different concentrations (30, 70 and 120 μg/mL) of compounds 2, 4, 5, 7, and 10, using ginsenoside Rg1 (120 μg/mL) as a positive control. (B) Quantitative analysis of the ISV index (number of intact vessels * 1+number of defective vessels * 0.5) in zebrafish treated with compounds 2, 4, 5, 7, and 10. Data represented as mean ± SEM. ## p < 0.01 compared to the control group; ** p < 0.01 compared to the PTK787 group. |
|
Results of anti-inflammatory activities. (A) Typical images on inflammatory sites in CuSO4-induced transgenic macrophages fluorescent of compounds 7, 8, 10, and 11, using ibuprofen (10 μM) as a positive control. (B) Quantitative analysis of the number of fluorescent macrophages. The data are represented as the mean ± SEM. ## p < 0.01 compared to the control group; * p < 0.05 and ** p < 0.01 compared to the CuSO4 group. |