- Title
-
Zebrafish Models of Craniofacial Malformations: Interactions of Environmental Factors
- Authors
- Raterman, S.T., Metz, J.R., Wagener, F.A.D.T.G., Von den Hoff, J.W.
- Source
- Full text @ Front Cell Dev Biol
|
|
|
|
|
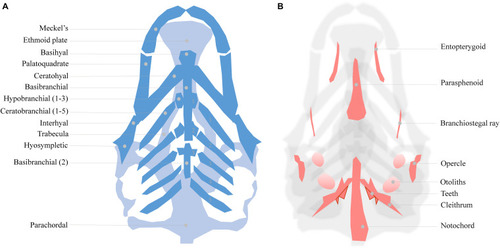
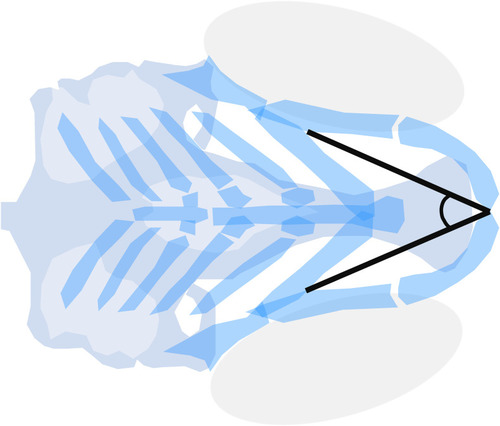
Meckel’s-palatoquadrate (M-PQ) angle is proposed as a reliable high-throughput standard parameter to assess craniofacial outcomes after single or mixed compound exposures. The measurements can be easily obtained by imaging of cartilage stained larvae and proved to be informative on a broad spectrum of craniofacial malformations. The M-PQ angle is especially affected in craniofacial malformations such as microcephaly and micrognathia. |
|
General summary showing the environmental factors smoking, alcohol use, vitamin imbalance, drug use, (xeno) estrogens and pesticides. These factors (represented in the upper part of the figure) differentially affect critical developmental processes such as the formation, survival, delamination, migration, condensation, and differentiation of CNCCs (represented in the lower part of the figure). Evidence of interactions between environmental factors that result in craniofacial malformations has been reported as well, these are indicated by black arrows. Exposure to environmental factors often results in aberrant signaling of essential pathways in craniofacial development including SHH, TGF, FGF, BMP, RA, and WNT. Moreover, gene mutations in these pathways can also interact with environmental factors, complicating the etiology. Known GxE interactions are indicated in this figure with red dotted arrows. |