- Title
-
A truncated Danio rerio PKZ isoform functionally interacts with eIF2alpha and inhibits protein synthesis
- Authors
- Liu, Z.Y., Jia, K.T., Li, C., Weng, S.P., Guo, C.J., and He, J.G.
- Source
- Full text @ Gene
|
Sequence analysis of DrPKZ-A and DrPKZ-B. (A) Expressions of DrPKZ-A and DrPKZ-B in zebrafish spleen. Zebrafish were challenged with ISKNV, and the spleens were collected after 6 days. RT-PCR was performed using primers covering the complete ORF. DrPKZ-A and DrPKZ-B were amplified at the predicted loci. (B) Domain organization is shown in a schematic manner. Numbers to the right represent the number of residues in DrPKZ-A, DrPKZ-B, and human PKR (HsPKR). Z-DNA binding domains (Zα and Zβ) are shown in green. Domains containing a dsRNA-binding motif are shown in light blue. The kinase domains of DrPKZ-A, DrPKZ-B (orange), and PKR (yellow) are labeled. (C) Comparison of the kinase domains from DrPKZ-A, DrPKZ-B, and HsPKR. The kinase subdomains are marked with Roman numerals as previously defined by Hanks and Hunter. The kinase insert domains (boxed in yellow), which are unique to eIF2α kinases, are located between kinase subdomains IV and V. Identical residues in the DrPKZs and in HsPKR are labeled with asterisks, and homologous residues are marked with tandem colons or single dots depending on their degree of homology. |
|
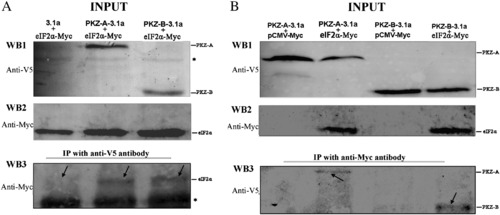
DrPKZ-A and DrPKZ-B interact with eIF2α. (A) Co-immunoprecipitation (co-IP) of co-expressed V5-tagged protein (3.1a, PKZ-A-3.1a or PKZ-B-3.1a) and eIF2α-Myc (Danio rerio eIF2α with a C-terminal Myc tag) in HEK293T cells. WB1 and WB2 show that all recombinant vectors are expressed normally. An IP was performed using mouse anti-V5 antibody followed by immunoblotting with a rabbit anti-Myc antibody. Compared to the control sample, eIF2α coprecipitated with DrPKZ-A and DrPKZ-B, as shown in WB3. (B) Reciprocal co-IP experiments showed that DrPKZ-A and DrPKZ-B interact with eIF2α. IP was performed using a rabbit anti-Myc antibody, followed by immunoblotting with a mouse anti-V5 antibody. All the recombinant vectors express normally in HEK293T cells (WB1 and WB2). When the lysates from the control cells groups (lanes 1 and 3) were precipitated with anti-Myc antibody, the corresponding V5-tagged DrPKZ-A or DrPKZ-B was not detected by anti-V5 antibody. However, when the lysates from cells co-transfected with eIF2α-Myc and PKZ-A-3.1 (lane 2) or from cells co-transfected with eIF2α-Myc and PKZ-B-3.1 (lane 4) were precipitated with anti-Myc antibody, V5-tagged DrPKZ-A or DrPKZ-B was detected by anti-V5 antibody. The asterisk “*” marks nonspecific bands. |
|
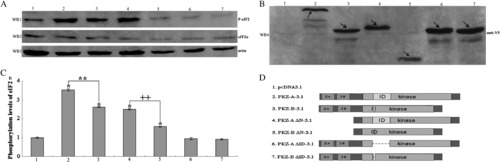
Effects of DrPKZ-A and DrPKZ-B on eIF2α phosphorylation. Lanes 1–7 represent cells co-transfected with the following recombinant vectors: pcDNA3.1a, PKZ-A-3.1a, PKZ-B-3.1a, PKZ-A ΔN-3.1a, PKZ-B ΔN-3.1a, PKZ-A ΔID-3.1a, PKZ-B ΔID-3.1a, and eIF2α-Myc. (A) The phosphorylation levels of eIF2α are shown in WB1, which was blotted with a rabbit anti-phospho-eIF2α (Ser51) antibody. Western blotting analysis for eIF2α and actin is shown in WB2 and WB3, which were blotted with rabbit anti-Myc and rabbit anti-actin antibodies, respectively. (B) Western blotting analysis to determine the expression of all recombinant vectors with mouse anti-V5 antibody. As indicated by the arrows, all recombinant vectors were expressed normally at the predicted molecular weights. (C) Quantification of the phosphorylated eIF2α levels versus actin levels. Compared with the control group (cells transfected with an empty vector) (column 1), the DrPKZ-A and DrPKZ-B-expressing cells display increases in the phosphorylation levels of eIF2α of approximately 3.5-fold and 2.6-fold, respectively (columns 2 and 3). DrPKZ-A ΔN and DrPKZ-B ΔN were less effective at phosphorylating eIF2α. Compared with the control group, these two truncated mutants elevate the phosphorylation levels of eIF2α by about 2.5-fold and 1.5-fold (columns 4 and 5). DrPKZ-A ΔID and DrPKZ-B ΔID lost nearly all of their kinase activity to eIF2α (columns 6 and 7). Data are presented as the means α S.D. (n = 3 replicates). * indicates a statistically significant difference relative to the pcDNA3.1a control (P < 0.01). ** indicates a statistically significant difference between DrPKZ-A and DrPKZ-B, and ++ indicates a statistically significant difference between DrPKZ-A ΔN and DrPKZ-B ΔN. |
|
The suppressive effects of DrPKZ constructs on the expression of a co-transfected GFP construct. HEK293T cells were co-transfected with pEGFP-N3 and DrPKZ constructs. Twenty-four hours after transfection, the expression levels of GFP were measured by fluorescence microscopy. All images were taken at random and exposed for the same time. |
Reprinted from Gene, 527(1), Liu, Z.Y., Jia, K.T., Li, C., Weng, S.P., Guo, C.J., and He, J.G., A truncated Danio rerio PKZ isoform functionally interacts with eIF2alpha and inhibits protein synthesis, 292-300, Copyright (2013) with permission from Elsevier. Full text @ Gene