- Title
-
Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish
- Authors
- Mosimann, C., Kaufman, C.K., Li, P., Pugach, E.K., Tamplin, O.J., and Zon, L.I.
- Source
- Full text @ Development
|
Cloning and characterization of the zebrafish ubi regulatory region. (A) Schematic of the zebrafish ubiquitin B locus zgc:172187 on linkage group 5, featuring a 71 bp non-coding first exon, a single 2 kb intron and a coding exon containing the ORF for a Ubiquitin octamer peptide. The red line indicates the PCR-amplified and subcloned 3.5kb ubi promoter fragment. (B-D′) EGFP fluorescence images of zebrafish injected with Tol2(–3.5ubi:EGFP) vector at the one-cell stage, (B) oblong to sphere stage (approx. 4 hpf), (C) 36 hpf, and (D,D′) adult. Note the transgene injection-derived mosaic EGFP expression that persists through all developmental stages compared with wild type. (E-G′) ubi:EGFP expression in stable transgenic lines. (E) Maternal EGFP contribution in embryos derived from outcrossing an F2 stable transgenic Tg(–3.5ubi:EGFP)F female to wild type compared with a transgene-negative sibling, here shown at the eight-cell stage (1.25 hpf). (F) ubi-EGFP expression at 48 hpf in offspring resulting from outcrossing of a F2 Tg(–3.5ubi:EGFP)F male to wt. (G,G′) Compound image overview of an adult zebrafish female heterozygous for Tg(–3.5ubi:EGFP)V, revealing widespread EGFP fluorescence including in the fins. wt, wild type. |
|
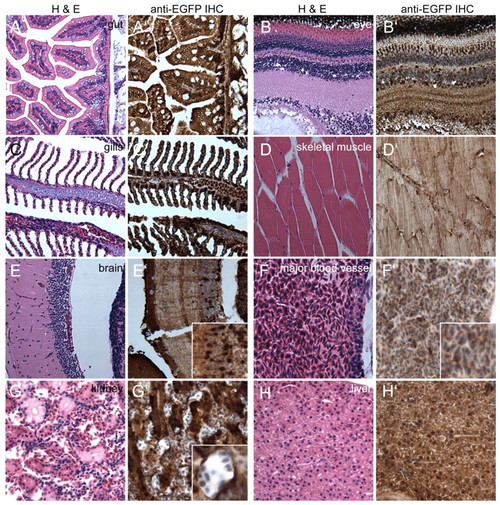
Sagittal sections through a heterozygous Tg(–3.5ubi:EGFP)V adult reveals widespread ubi expression in internal organs. (A-H′) Sagittal sections of a PFA-fixed heterozygous Tg(–3.5ubi:EGFP)V adult zebrafish female stained with H&E. Adjacent sections are stained for EGFP expression through IHC (brown stain). (E′,F′,G′) Insets show magnified areas to illustrate cellular details and variations in EGFP expression levels in cortical neurons (E′) and kidney tubule cells (G′). (F′) Major blood vessel illustrating EGFP-positive erythrocytes as the only unambiguously identifiable blood cell type. |
|
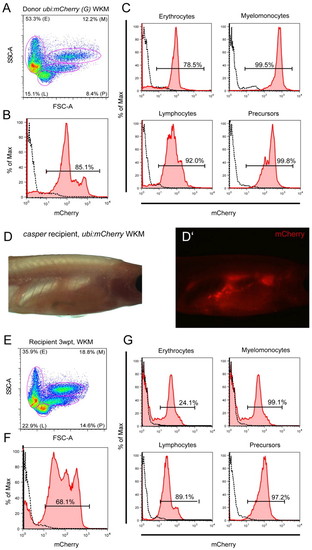
Whole kidney marrow transplantation using ubi:mCherry. (A-C) Cells isolated from Tg(–3.5ubi-mCherry)G adult WKM were transplanted via retro-orbital injection into irradiated casper recipient adults and read-out 3 weeks post transplant (wpt) for mCherry blood chimerism by fluorescence microscopy (D,D′) and flow cytometry (E-G) of isolated recipient WKM. mCherry-positive and thus donor-derived cells efficiently engrafted recipients and contributed to all resolvable blood populations (G). |
|
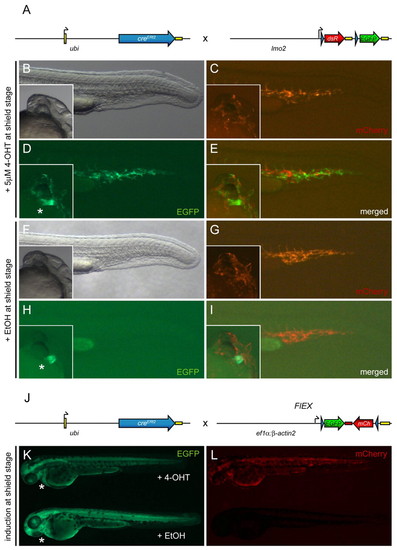
Tg(–3.5ubi:creERt2;cmlc2-EGFP) provides 4-OHT-inducible ubiquitous Cre activity. Asterisks indicate cmlc2-EGFP expression in the heart, which segregates with the ubi:creERt2 transgene. (A) Schematic of the ubi:creERt2 transgene and lmo2:lox-dsRed-lox-EGFP (lmo2:Switch) reporter cross shown in panels (B-I). Black arrows indicate transcription start positions, yellow boxes are non-coding 52 and 32 UTR/polyA sequences, blue arrow heads indicate loxP sites. (B-I) Images depict the posterior region of live ubi:creERt2; lmo2:Switch double-positive zebrafish embryos at approximately 24 hpf, with insets showing cmlc2:EGFP expression to confirm presence of both transgenes. Embryos were induced at shield stage (6 hpf) with 5 μM 4-OHT (B-E) or ethanol (EtOH) carrier (F-I). mCherry expression marks lmo2:Switch-expressing cells in endothelial cell populations of the tail and head (C,G), whereas EGFP reveals specific 4-OHT dependent loxP cassette excision from lmo2:Switch by Cre (D,E), which did not occur in ethanol controls (H,I). (J) Schematic of the ubi:creERt2 transgene and Tg(eab2:[EGFP-T-mCherry]) (FlEX) reporter cross shown in K and L. Note that FlEX is driven by a compound ef1α:β-actin2 promoter fragment which drives a bi-directional Terminator (T)-separated EGFP/mCherry cassette that inverts to enable mCherry expression after Cre-mediated recombination (Boniface et al., 2009). (K,L) Live ubi:creERt2; FlEX double-positive zebrafish embryos at approximately 3 dpf. The embryo on top was treated with 5 μM 4-OHT at shield stage, whereas the embryo at the bottom was treated with ethanol carrier control. EGFP fluorescence indicates default FlEX reporter expression (K), whereas mCherry fluorescence (L) reveals successful Cre-mediated loxP recombination, which inverts the EGFP-T-mCherry cassette in FlEX and triggers mCherry expression. |
|
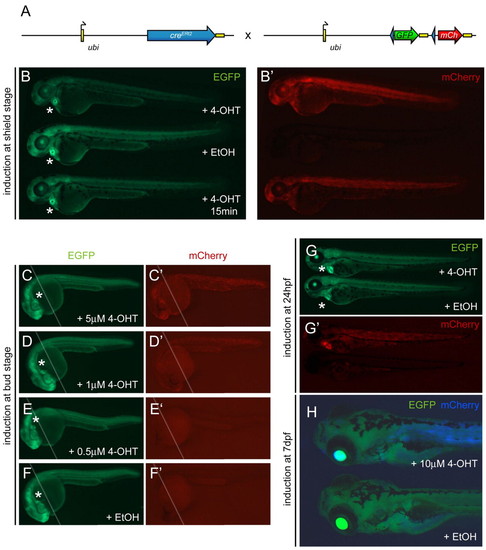
ubi:Switch is a ubiquitous lineage-tracing transgene. The asterisks indicate heart-specific cmlc2-EGFP expression marking the ubi:creERt2 transgene. (A) Schematic of the ubi:creERt2 transgene and Tg(–3.5ubi:loxP-GFP-loxP-mCherry) (ubi:Switch) reporter cross. Arrows indicate transcription start positions, yellow boxes are non-coding 5′ and 3′ UTR/polyA sequences, blue arrowheads indicate loxP sites. (B,B′) Imaging of live ubi:creERt2; ubi:Switch double-positive zebrafish embryos at approximately 3 dpf for baseline GFP expression (B) and mCherry indicating successful loxP excision (B′). Embryos were treated at shield stage (6 hpf) with 5 μM 4-OHT, ethanol (EtOH) carrier, or a short 15 minute pulse of 5 μM 4-OHT (top to bottom). Both 4-OHT treatments induce strong mCherry expression (B′), with apparent GFP fading in the longer 4-OHT treatment (B, top), compared with ethanol control (B,B′, center). (C-F′) Approximately 30 hpf zebrafish embryos double-positive for ubi:creERt2; ubi:Switch were treated with 4-OHT or ethanol control at bud stage as indicated and compound imaged for GFP fluorescence (C-F) to visualize ubi:Switch default expression or mCherry fluorescence (C′-F′) to reveal successful loxP excision (the white line indicates merge of the anterior and posterior focal planes). Whereas 5 μM 4-OHT potently induces loxP recombination (C′), and 1 μM 4-OHT causes notably reduced mosaicism (D′), 0.5 μM 4-OHT fails to induce a widespread response (E′) similar to the ethanol control (F′). (G-H) ubi:creERt2; ubi:Switch embryos 5 μM 4-OHT or ethanol control treated at 24 hpf (G,G′) or 7dpf (H) reveal 4-OHT-dependent loxP recombination and de novo mCherry expression from the ubi:Switch transgene. Note the strong mCherry expression in the heart in the 4-OHT-treated larva (G′, top), suggesting promoter cross-talk between the cmlc2 marker driver and ubi:creERt2. Because of their drastically increased size, the embryos induced at 7dpf (H) were treated with increased dose (10 μM) of 4-OHT or ethanol control and visualized for GFP (green) and mCherry expression (blue) channel. |