- Title
-
Nicotine response genetics in the zebrafish
- Authors
- Petzold, A.M., Balciunas, D., Sivasubbu, S., Clark, K.J., Bedell, V.M., Westcot, S.E., Myers, S.R., Moulder, G.L., Thomas, M.J., and Ekker, S.C.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
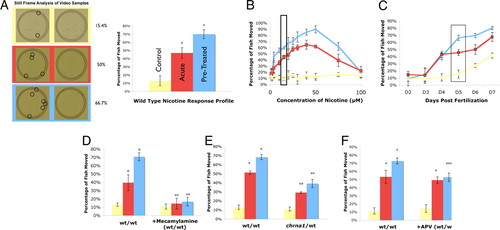
Nicotine response profiling of zebrafish larvae. Locomotion of zebrafish larvae was assessed using sibling animals. Yellow datasets (Control) indicate basal locomotion rates (no stimulation). Red datasets (Acute) demonstrate locomotion rates during a single exposure to nicotine. Blue datasets (Pretreated) indicate locomotion rates during nicotine exposure in fish that had been pretreated with a single, brief exposure to nicotine 8 h prior (see Experimental procedures). All data points represent at least three independent measurements on independent biological samples with at least 10 animals measured for each replicate. (A) Representative data for standard nicotine response profile conditions. Video imaging of a single behavioral analysis is shown at left. Movement rates were determined as a function of differential location (circled fish) during a standard time window of 0.25 s (middle). A standard wild-type response profile of 5-day-old zebrafish is shown (right); this profile examines normal movement rates (Control), movement as a function of a single nicotine treatment (Acute), and shows the increased locomotor activity over an acute response after receiving multiple doses of nicotine (Pretreated). (B) Dose–response of 5-day-old zebrafish to nicotine. Zebrafish show an increase in movement for doses from 2.5–50 μM doses of nicotine. The greatest difference in movement between the acute and pretreated fish occurs in doses between 10 and 25 μM; the main dose used for the subsequent work (10 μM) is highlighted by the black box. Note the inverted-U-shape of the curve, with animals showing reduced movement enhancement at doses above 50 μM nicotine. (C) Zebrafish respond to nicotine at 4 days of age and not before. Zebrafish can be sensitized to nicotine at 5 days of age and not before, highlighted by the black box. (D) Mecamylamine, a central nervous system specific nicotinic competitive inhibitor, quenches the overall nicotine response when added before the testing dose of nicotine without affecting the basal locomotion rate in the absence of nicotine. (E) chrna1 heterozygous fish exhibit a reduced nicotine response when compared to their wild-type siblings. (F) APV, an NMDAR competitive inhibitor, quenches the development of the sensitization response when added concurrently with initial nicotine dose. *, P < 0.05 when comparing to control or acute. **, P < 0.05 when comparing to corresponding treatment group. ***, P < 0.05 when comparing to control, but not acute. Also P < 0.05 when comparing to the corresponding treatment group. PHENOTYPE:
|
|
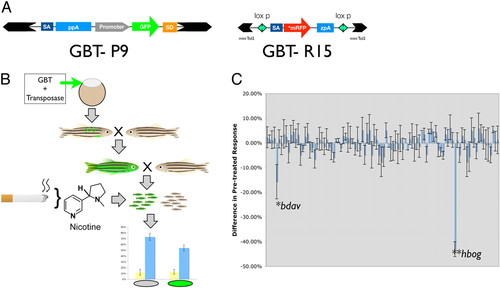
Zebrafish behavioral screen for nicotine response mutants. (A) Diagram of gene-breaking transposons (GBTs) deployed in this study. GBT-P9 [this Tol2-based vector is adapted from the GBT-P6 vector from (28)] harbors a transcriptional terminator cassette in cis with a strong ubiquitous promoter driving the green fluorescent protein (GFP) reporter upstream of a splice-donor sequence. Intronic insertions in the sense orientation activate the GFP reporter by providing a functional poly(A) signal from the nearby genetic locus. GBT-R15 contains a modified transcriptional terminator cassette that harbors an AUG-free monomeric red fluorescent protein (mRFP) reporter cassette. mRFP expression is only detected after an in-frame integration event. GBT-R15 also deploys the miniTol2 gene vector backbone and is flanked by loxP sites for downstream genetic causality analyses. (B) Behavioral genetic screening paradigm using GBTs as insertional mutagens. Standard transposase-mediated transgenesis protocols (20) generate a pool of mosaic F0 founder animals. These are outcrossed to generate stable transgenic F1 animals that are then outcrossed to generate mixed clutches of wild-type and mutant siblings. Mutant animals are segregated from their genetically wild-type siblings using fluorescent tags in these GBTs (GFP fluorescence is diagrammed as noted in the GBT-P9 screen; for the GBT-R15 screen, red fluorescence was used to sort). Nicotine response profile is obtained on each subpopulation (fluorescently tagged vs. wild-type). Nicotine was administered at the standard dosage of 10 μM. (C) Differential nicotine response profile between wild-type and fluorescently tagged siblings are diagrammed. Response bars are derived by subtracting the pretreated response of fluorescently tagged GBT-mutants from the pretreated response of their wild-type siblings. This analysis identified two nicotine response mutants (*, P j 0.05), bette davis (bdav) and (**, P j 0.05) humphrey bogart (hbog), out of a total of 102 lines screened: 89 GBT-P9 representing an estimated 178 expressed loci and 23 GBT-R15 representing an estimated 35 expressed loci. PHENOTYPE:
|
|
bette davis (bdav) Nicotine response mutation is linked to a GBT insertion in zebrafish chaperone containing protein 8 (cct8). (A) The founder F1 animal (Fig. 2C) harbored three independently expressed GBTs. Upon out-crossing and further analysis, animals with altered nicotine response profile (A) harboring only a single GFP locus were isolated and propagated for six generations formally isolating the bdav nicotine response locus. Nicotine was administered at the standard dose of 10 μM. (B) bdav heterozygous fish show no difference in response to allyl isothiocyanate or extreme temperatures when compared to their wild-type siblings. (C) The single expressed GFP locus is encoded by a GBT integration in predicted intron two of zebrafish chaperone containing protein 8 (cct8). (D) RT-PCR analysis of cct8 transcript in wild-type, heterozygous, and homozygous bdav embryos. Primers in exons 1 and 5 were used to detect wild-type transcript of the cct8 gene. Primers in exon 5 and the GFP portion of the transposon were used to determine expression of transposon mRNA. RT-PCR using primers in β-actin were performed as an internal control. (E) Sagittal imaging of a whole mount in situ hybridization for cct8 at 1 day post fertilization (pf) (top) through 5 days pf (bottom) shows high levels of neural expression. (F) Phylogenetic analysis (Ensembl) indicates cct8 is an evolutionarily ancient gene and is encoded by a single ortholog in humans and other mammals. *, P < 0.05 when comparing to control or acute. **, P < 0.05 when comparing to corresponding treatment group. |
|
humphrey bogart (hbog) Nicotine response mutation is encoded by the zebrafish Gaba-B receptor 1.2 (gabbr1.2) locus. (A) Sagittal fluorescent imaging (anterior to left) shows a general neural expression of mRFP-gabbr1.2 fusion protein at 5 days pf. (B) Sagittal imaging (anterior to left) of a whole mount in situ hybridization for gabbr1.2 at 5 days pf (bottom) shows high levels of neural expression. (C) Full nicotine response profile of the hbog mutant comparing homozygous and heterozygous mutant animals to wild-type siblings at the standard nicotine dosage of 10 μM. Reduction from wild-type response is seen in both acute and sensitized groups. Reduction of homozygote phenotype is greater than heterozygote phenotype (P = 0.05). (D) hbog heterozygous fish show no difference in response to noxious chemicals (allyl isothiocyanate) or temperatures (4 and 42 °C) when compared to wild-type siblings. (E) Schematic representation of the GBT-R15 GBT insertion in intron 6 of the zebrafish gabbr1.2 gene. Location of exons 4 and 9 of the gabbr1.2 gene and primers A, B, and C used for RT-PCR analysis are indicated. (F) RT-PCR analysis of gabbr1.2 transcript in wild-type, heterozygous, and homozygous hbog fish. Primers in exons 4 and 9 were used to detect wild-type transcript of the gabbr1.2 gene. RT-PCR using primers in β-actin were performed as an internal control. (G) Germline propagation of Cre-mediated reversion of GBT-induced hbog mutant shows a nicotine response profile indistinguishable from wild-type sibling animals. (H) Simplified homology of the gabbr1/hbog locus shown in red. Humans and other mammals encode a single gabbr1 locus. Derived from Ensembl homology engine. *, P < 0.05 when comparing to control or acute. **, P < 0.05 when comparing to corresponding wild-type group. ***, P < 0.05 when comparing to corresponding heterozygote group and wild-type group. |