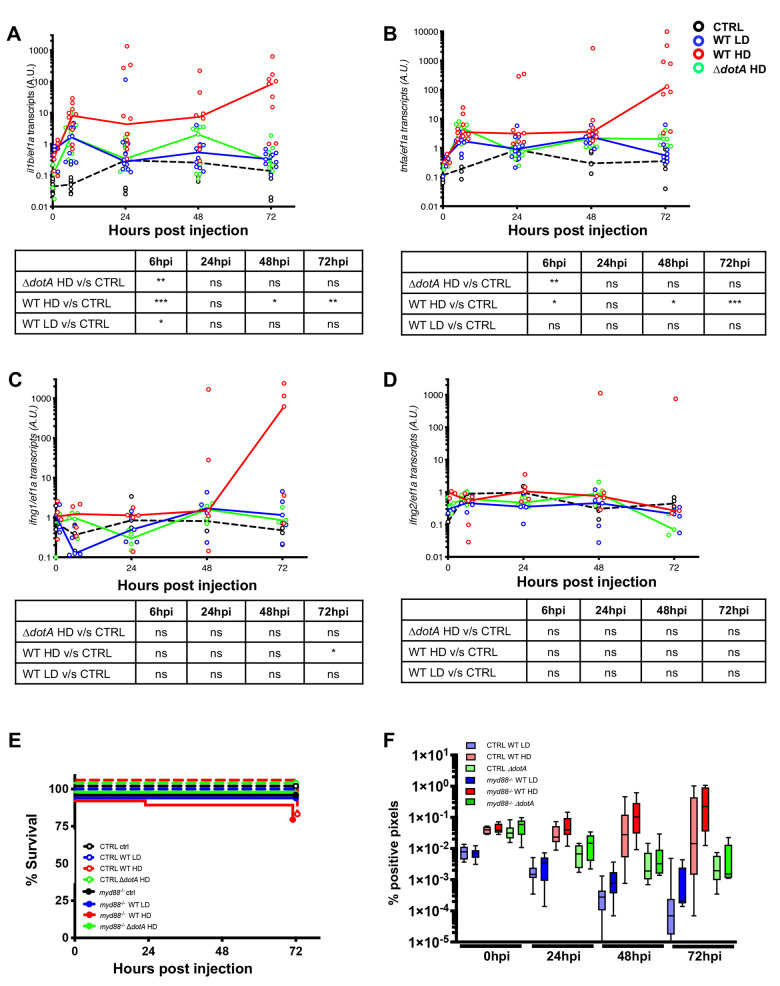
Fig 7
A-D) Cytokine gene (il1b, tnfa, ifng1, ifng2) induction was measured from non-injected larvae as control (CTRL, dashed black curves) and individual zebrafish larvae injected with a LD (blue curves) or a HD (red curves) of WT-GFP, or a HD of ΔdotA-GFP (green curves). Data plotted are from 2 pooled experiments (n = 10 larvae for each condition) for il1b and tnfa, and from 1 experiment (n = 5 larvae for each condition) for ifng1 and ifng2; individual values are shown, and curves correspond to the medians. Statistical analyses are shown in the table under each graph. E) Survival curves of CTRL zebrafish larvae injected with WT-GFP Low Dose (LD) (blue dashed curve) or High Dose (HD) (red dashed curve), or with ΔdotA -GFP HD (green dashed curve), and myd88hu3568 mutant zebrafish larvae injected with WT-GFP LD (blue curve) or HD (red curve), or with ΔdotA -GFP HD (green curve). Non-injected CTRL larvae (black dashed curves), and myd88hu3568 mutant larvae (black curves) were used as control. Infected and control larvae (n = 72 fish for myd88hu3568 mutant conditions and n = 57 fish for CTRL conditions) were incubated at 28°C. Data plotted are from 3 pooled independent experiments. F) Bacterial burden evaluation by quantification of % of fluorescent pixel counts on individual injected larvae followed over time from 0 to 72 hpi. Each larva was imaged daily, and images were analysed with Fiji for bacterial burden evaluation. Two pooled experiments, 8 larvae for each condition. P < 0.05 was considered statistically significant (symbols: **** P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05). No symbol on graphs means that not statistically differences were observed.