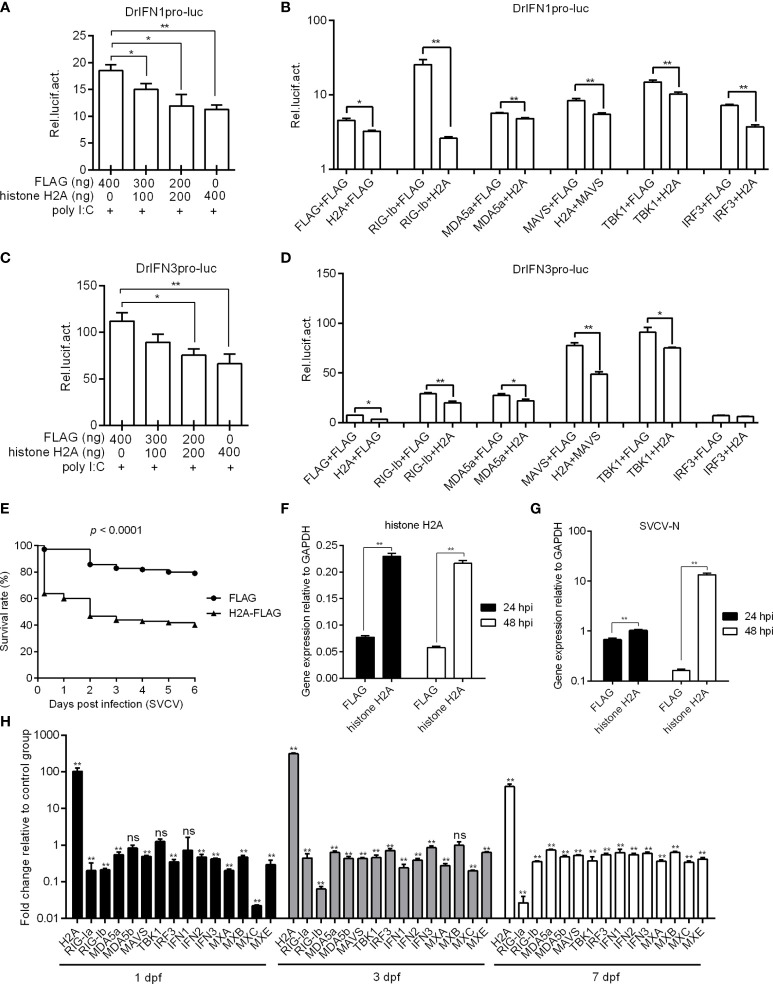
Fig. 2
Zebrafish histone H2A negatively regulates RLR signaling. (A) Effect of zebrafish histone H2A on the IFN1 promoter activity mediated by poly I:C stimulation. (B) Effect of zebrafish histone H2A on the IFN1 promoter activity mediated by RLR signaling pathway. (C) Effect of zebrafish histone H2A on the IFN3 promoter activity mediated by poly I:C stimulation. (D) Effect of zebrafish histone H2A on the IFN3 promoter activity mediated by RLR signaling pathway. For (A, C), EPC cells transfected with various indicated plasmids and DNA concentration were stimulation with poly I:C. Another 24 h later, the cells were lysed and used for luciferase activity assay. For (B, D), EPC cells were transfected with the indicated combination. At 48 h post-transfection, the cells were used for luciferase activity assay. (E) Effect of zebrafish histone H2A on the larvae survival. (F) The confirmation of zebrafish histone H2A expression in the histone H2A-overexpressed larvae with SVCV infection. (G) Effect of zebrafish histone H2A on the expression of SVCV-N gene. For (E–G), zebrafish larvae microinjected with p3×FLAG or H2A-FLAG were infected with 2×106 PFU/mL SVCV for 24 h. For (E), larvae were monitored for 6 days. For (F, G), larvae were collected at 24 and 48 hpi, and used for qRT-PCR. (H) Effect of zebrafish histone H2A on the expression of antiviral genes involved in RLR signaling pathway. Zebrafish larvae microinjected with p3×FLAG or H2A-FLAG were collected at 1, 3 and 7 dpf, and used for qRT-PCR. For (A–D, F–H), data represented means ± SEM (n = 3), and were tested for statistical significance. *p < 0.05; **p < 0.01; ns, not significant.