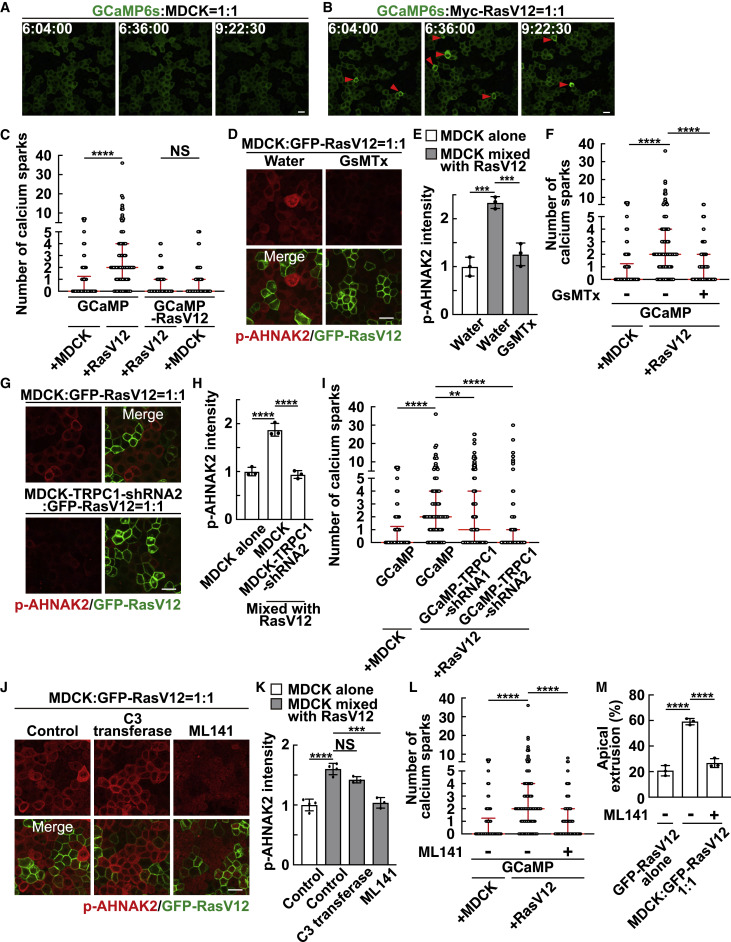
Fig. 2 Figure 2. Calcium sparks are induced in normal cells mix cultured with RasV12 cells via the mechanosensitive calcium channel TRPC1 (A and B) Time-lapse analysis of calcium sparks in GCaMP-expressing normal cells mix cultured with normal cells (A) or RasV12-transformed cells (B). Representative images extracted from time-lapse imaging of MDCK-GCaMP6s cells mix cultured with normal MDCK cells (A) or MDCK-pTRE3G Myc-RasV12 cells (B) at a ratio of 1:1 during 6–10 h after the induction of RasV12 expression. The red arrowheads indicate calcium spark-positive cells. (C) Quantification of the number of calcium sparks in MDCK-GCaMP6s cells or MDCK-pTRE3G Myc-RasV12-GCaMP6s cells mix cultured with normal MDCK or MDCK-pTRE3G Myc-RasV12 cells at a ratio of 1:1 during 6–10 h after the induction of RasV12 expression. Cumulative data from 3 (GCaMP) or 2 (GCaMP-RasV12) independent experiments are shown as medians ± IQRs (interquartile ranges). ∗∗∗∗p < 0.0001 and NS, not significant (Kruskal-Wallis test with Dunn’s test); n = 30 cells for each experiment. (D and E) Effect of the mechanosensitive calcium channel inhibitor GsMTx on AHNAK2 phosphorylation. (D) Immunofluorescence images of p-AHNAK2 (red) in the absence or presence of GsMTx. (E) Quantification of the fluorescence intensity of p-AHNAK2. Values are expressed as a ratio relative to the average of MDCK alone (water). Data are means ± SDs from 3 independent experiments. ∗∗∗p < 0.001 (1-way ANOVA with Dunnett’s test); n = 50 cells for each experiment. (F) Effect of GsMTx on the frequency of calcium sparks. Cumulative data from 3 independent experiments are shown as medians ± IQRs. ∗∗∗∗p < 0.0001 (Kruskal-Wallis test with Dunn’s test); n = 30 cells for each experiment. (G and H) Effect of TRPC1-knockdown on AHNAK2 phosphorylation. (G) Immunofluorescence images of p-AHNAK2 (red). (H) Quantification of the fluorescence intensity of p-AHNAK2. Values are expressed as a ratio relative to the average of MDCK alone. Data are means ± SDs from 3 independent experiments. ∗∗∗∗p < 0.0001 (1-way ANOVA with Dunnett’s test); n = 50 cells for each experiment. (I) Effect of TRPC1-knockdown on the frequency of calcium sparks. MDCK-GCaMP6s-TRPC1-shRNA1 or -shRNA2 cells were mix cultured with MDCK-pTRE3G Myc-RasV12 cells at a ratio of 1:1. Cumulative data from 4 (GCaMP-TRPC1-shRNA1) or 3 (the other conditions) independent experiments are shown as medians ± IQRs. ∗∗p < 0.01 and ∗∗∗∗p < 0.0001 (Kruskal-Wallis test with Dunn’s test); n = 30 cells for each experiment. (J and K) Effect of the Rho inhibitor C3 transferase or the Cdc42 inhibitor ML141 on AHNAK2 phosphorylation. (J) Immunofluorescence images of p-AHNAK2 (red) in the absence or presence of C3 transferase or ML141. (K) Quantification of the fluorescence intensity of p-AHNAK2. Values are expressed as a ratio relative to the average of MDCK alone (control). Data are means ± SDs from 4 (control) or 3 (C3 transferase and ML141) independent experiments. ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, and NS, not significant (1-way ANOVA with Dunnett’s test); n = 50 cells for each experiment. (L) Effect of ML141 on the frequency of calcium sparks. Cumulative data from 3 independent experiments are shown as medians ± IQRs. ∗∗∗∗p < 0.0001 (Kruskal-Wallis test with Dunn’s test); n = 30 cells for each experimental condition. (M) Effect of ML141 on apical extrusion of RasV12-transformed cells. At 24 h after RasV12 induction, the ratio of the apical extrusion was analyzed. Data are means ± SDs from 3 independent experiments. ∗∗∗∗p < 0.0001 (1-way ANOVA with Dunnett’s test); n > 250 cells for each experiment. (A, B, D, G, and J) Scale bars, 20 μm. See also Figure S3; Videos S1, S2, and S3.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Cell Rep.