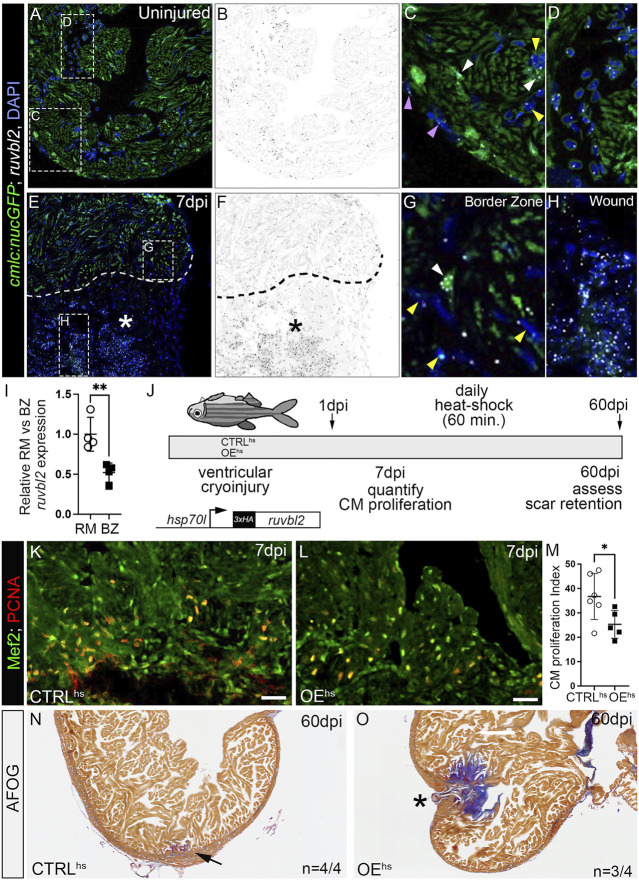
FIGURE 4
ruvbl2 suppresses cardiomyocyte proliferation during zebrafish cardiac regeneration and leads to scar retention. (A–H) RNAScope in situ hybridization of ruvbl2 transcripts in representative cardiac sections from uninjured or 7 dpi hearts immunostained for GFP and counterstained with DAPI. Ruvbl2 fluorescent signals in (A,E) are pseudocolored to black and white in (B,F). Boxed regions in A and E are shown in magnified views in (C,D,G,H) as indicated. (C,G) Purple arrowhead, epicardial cells; white arrowhead, myocardial cells; yellow arrowhead, endocardial cells. (D) Ruvbl2 expression in circulating cells. (E,F) Dotted line shows the border between the wound (marked with an asterisk) and the spared cardiac tissue. (I) Quantification of relative ruvbl2 expression between the remote myocardium (RM) and border zone (BZ). (J) Schematic of experimental design for regeneration experiments. Adult zebrafish carrying the Tg (hsp70l:ruvbl2) overexpression transgene were given a single heat shock per day following cryoinjury to the ventricle. Experimental endpoints were at 7 dpi and 60 dpi to assay cardiomyocyte proliferation and regeneration, respectively. (K,L) Sections of WT and Tg (hsp70l:ruvbl2) hearts immunostained for Mef2c and PCNA at 7 dpi. (M) Quantification of the cardiomyocyte proliferation index at 7 dpi. (N,O) Representative sections of WT and Tg (hsp70l:ruvbl2) hearts stained with acid fuchsin orange G (AFOG) at 60 dpi. Black arrow highlights regenerated wound area. Black asterisk highlights fibrotic scar tissue with open myocardial wall. 4 of 4 WT hearts regenerated well and 3 of 4 OEhs hearts retained substantial scar tissue as shown. Mean ± SD are shown. *p < 0.05; **p < 0.01. Scale bars: 25 μm.