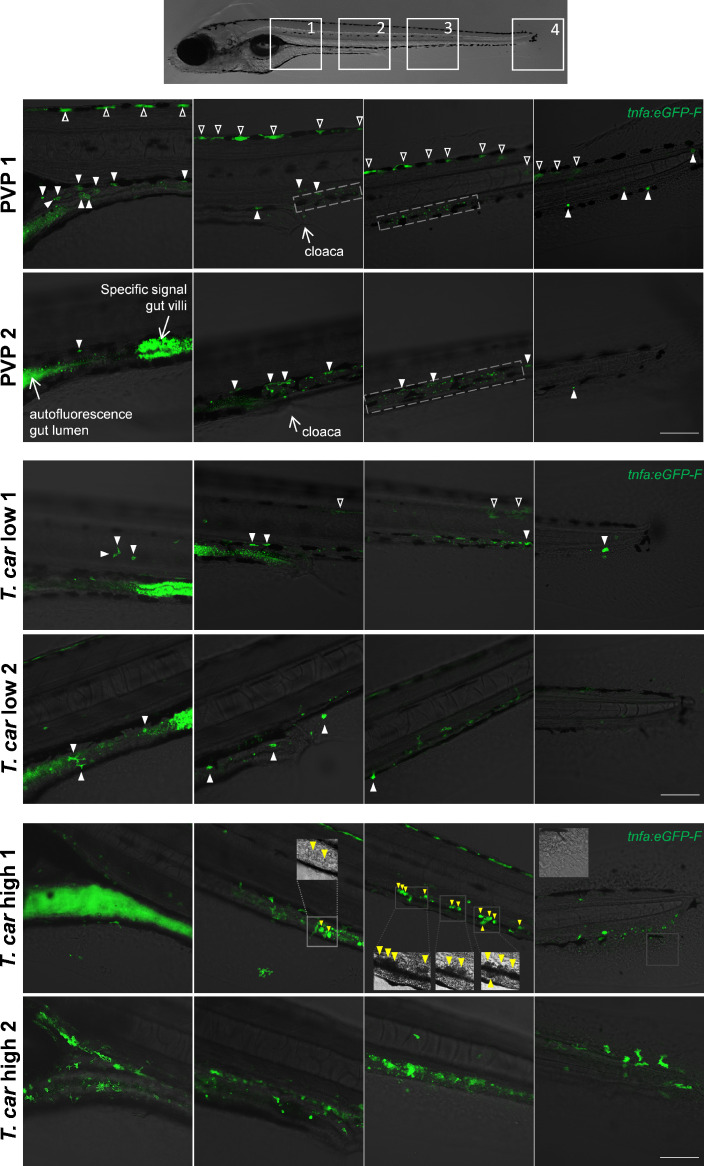
Figure 9—figure supplement 1. Tg(tnfa:eGFP-F) zebrafish larvae (5dpf), were injected intravenously with n = 200 T. carassii or with PVP. At 4 dpi, larvae were separated in high- and low-infected individuals and n = 3–6 fish per group were imaged with a Zeiss lsm-510 Confocal Microscope using ×10 magnification. Images were acquired using the same settings, thus allowing direct comparison of the intensity of the eGFP signal. Representative images of four different locations along the entire trunk and tail region of two individuals per group are shown. Each image has a frame size of 2048 × 2048 pixels allowing for enlargement of the images, if needed, for a more detailed view. Scale bar indicates 100 μm. Upper panels: in non-infected (PVP) individuals, low constitutive tnfa:eGFP expression can be observed in skin keratinocytes (open arrowheads) along the dorsal side of the larva as well as the flank skin depending on the orientation of the larva and focus (area 3–4 of PVP1); constitutive tnfa:eGFP expression is also detected in endothelial cells of the caudal vein (dashed square) as also reported in Figures 8–10. Stronger constitutive expression is observed in enterocytes of gut villi and this could vary between individuals and focus plane of the image. Autofluorescence of the gut lumen is a common feature and can vary per individual depending on the degree of absorption of the yolk sac or time of feeding (see also Figure 4). Finally, constitutive tnfa:eGFP expression can be detected in leukocytes (white arrowheads) distributed along the major trunk vessels, recognizable by their typical polymorphic morphology. Middle and lower panels: to allow maximum visibility of the overall tnfa:eGFP expression in high- and low-infected individuals, we limited the number of symbols added to each image; at this magnification it would render the images very crowded and we refer the reader to the legend of the upper panels (PVP). In low-infected individuals (T. car low), the number of tnfa:eGFP + leukocytes was only marginally higher than that in non-infected individuals (see also Figure 10C); leukocytes were distributed along the trunk, mostly lining the major blood vessels and in the tail tip loop, or could be seen in the peritoneal cavity around the gut area; only few leukocytes clearly displayed a stronger eGFP signal than observed in PVP individuals (only these are indicated by white arrowheads). This supports the previous observation (Figures 8–10) that low-infected individuals display a moderate inflammatory response. In high-infected individuals (T. car high), not only the number of tnfa:eGFP + cells but also the intensity of their eGFP signal was higher than that observed in PVP and low-infected individuals. Only in high-infected individuals, foamy macrophages strongly positive for tnfa:eGFP expression (yellow arrowheads) were seen along the major vein (posterior cardinal vein before the cloaca and caudal vein after the cloaca) and were also clearly recognizable in bright-field images by their dark and granular appearance (insets in area 2–3, T. car high 1). In high-infected individuals, numerous leukocytes strongly positive for tnfa:eGFP expression were observed also in the peritoneal cavity around the gut area and in the tail tip along the vessels or in the fins, altogether confirming the overall high inflammatory response in these individuals also reported in Figure 10. Note the high number of parasites extravasated in the tissue, recognizable by their typical ‘worm-like’ morphology (inset area 4) in the tail fin of high-infected individual number 1.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Elife