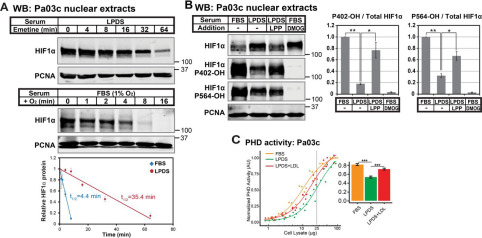
Fig. 3 Figure 3. Lipoproteins regulate HIFα stability by controlling HIFα prolyl hydroxylation. A, immunoblots (WB) of nuclear extracts from Pa03c cells cultured in LPDS for 16 h prior to treatment with the translation inhibitor emetine (25 μm) for the indicated time (top) or Pa03c cells cultured in FBS at 1% O2 for 4 h and then shifted to normoxia for the indicated time (bottom). HIF1α was normalized to PCNA signal and plotted relative to the t = 0 time point. Linear regression curves were used to calculate HIF1α t½ (n = 3, mean ± S.E. (error bars)). B, immunoblots of nuclear extracts from Pa03c cells cultured for 14 h in FBS or LPDS with the following additions: bovine lipoproteins (LPP; 1 mg/ml) or the PHD inhibitor DMOG (1 mm), followed by the addition of MG132 (10 μm) to all conditions for an additional 2 h. HIF1α signal was normalized to PCNA, and the level of hydroxylated HIF1α relative to total was normalized to that in FBS (n = 3, mean ± S.E.). p values from a single-column t test (LPDS versus FBS) or Student's t test (paired, LPDS + LPP versus LPDS) are shown; *, p < 0.05; **, p < 0.005. C, PHD activity assay of cell lysates from Pa03c cells cultured in FBS, LPDS, or LPDS with human low-density lipoprotein (1 mg/ml) for 16 h. Four-parameter log logistic models were fit to data obtained from at least three independent experiments. A bar plot shows the calculated PHD activities from the curves at 25 µg. ***, p < 0.0005; Student's t test.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ J. Biol. Chem.