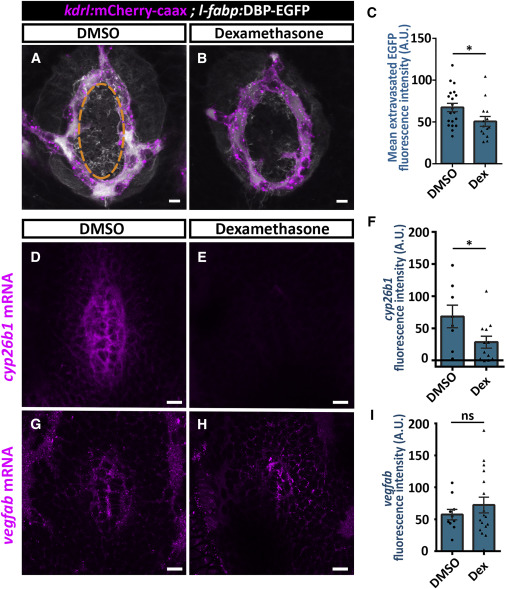
Fig. 6 Dexamethasone Treatment Decreases Hypophyseal Permeability and cyp26b1 Expression (A–C) Measurement of hypophyseal permeability using the transgenic biosensor Tg(l-fabp:DBP-EGFP;kdrl:mCherry-caax) was performed as described in Figure 4. Larvae were treated with DMSO (A, n = 20) or dexamethasone at 100 μM (B, n = 14) between 4 and 5 dpf. Dexamethasone treatment led to reduced extravasation of DBP-EGFP from the hypophyseal loop (C, ∗p < 0.05; Student’s t test). A.U., arbitrary units. Scale bar: 5 μm. (D–I) Measurement of in situ cyp26b1 and vegfab expression upon dexamethasone treatment. Confocal z stack images showing fluorescent in situ hybridization (FISH) of transgenic larvae using probes directed against cyp26b1 (D and E) or vegfab mRNAs (G and H). Larvae were treated with DMSO or dexamethasone at 100 μM between 4 and 5 dpf, followed by FISH, and the ISH fluorescence in the neurohypophyseal area was quantified. Dexamethasone treatment led to reduced ISH fluorescence of cyp26b1 (E) but not of vegfab (H). (F; ∗p < 0.05; Student’s t test; n = 8, 12 for each treatment. I; ns = not significant; n = 10, 17). A.U., arbitrary units. Scale bar: 10 μm. Data are presented as mean ± SEM (C, F, and I). See related Figures S5–S7.
Reprinted from Developmental Cell, 47(6), Anbalagan, S., Gordon, L., Blechman, J., Matsuoka, R.L., Rajamannar, P., Wircer, E., Biran, J., Reuveny, A., Leshkowitz, D., Stainier, D.Y.R., Levkowitz, G., Pituicyte Cues Regulate the Development of Permeable Neuro-Vascular Interfaces, 711-726.e5, Copyright (2018) with permission from Elsevier. Full text @ Dev. Cell