Fig. 4
Bace2 Regulates Melanophore Dendricity via PI3K/mTOR Signaling
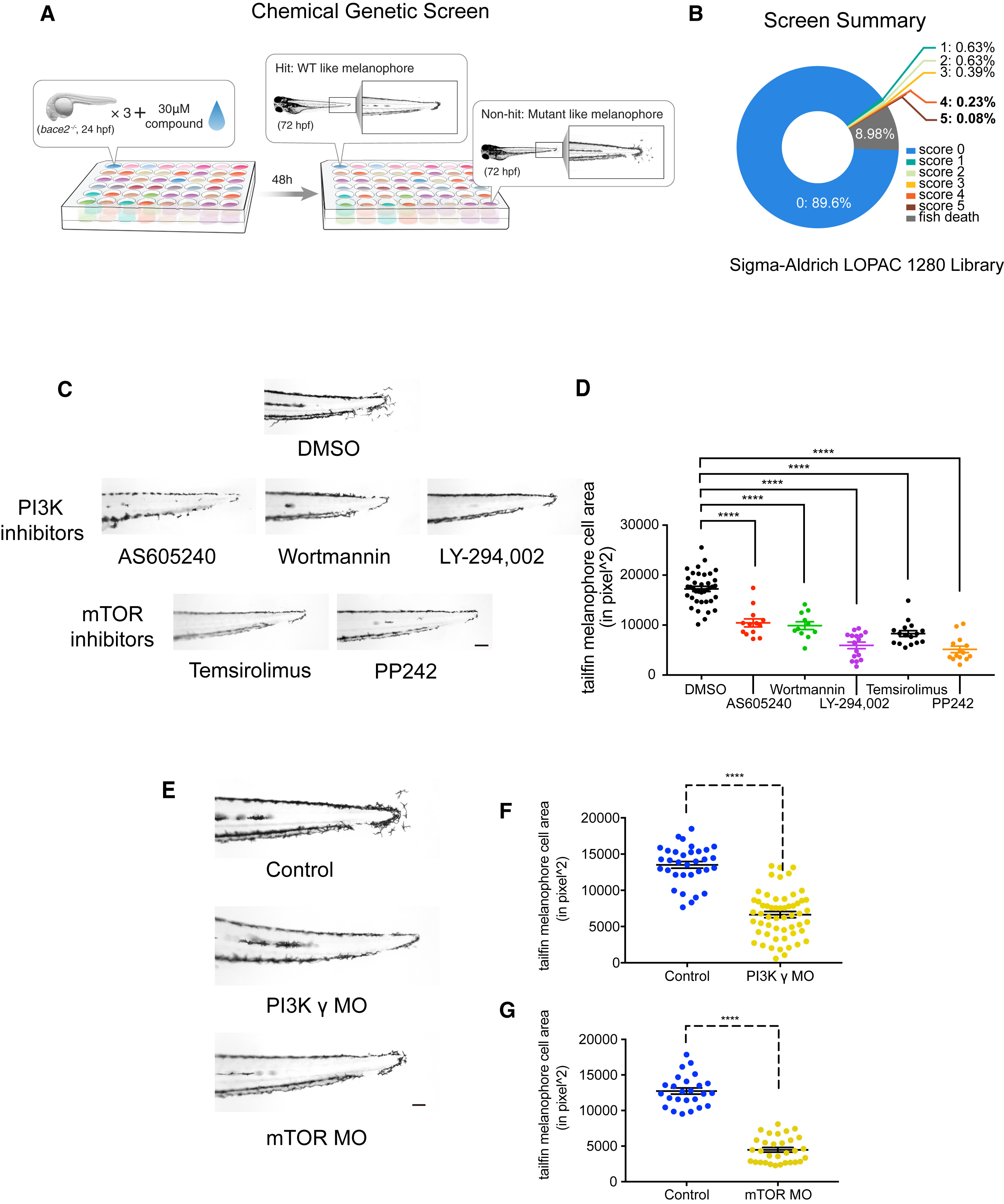
(A) Scheme for chemical suppressor screen of the bace2−/− mutant; 24 hpf bace2−/− embryos were treated with each compound from the Sigma LOPAC 1280 library at 30 μM for 48 hr, in order to identify chemicals that could rescue the melanophore defects.
(B) Compounds were scored with a range of 0 (non-rescued, mutant-like) to 5 (fully rescued, WT-like).
(C) Top hits from the screen (with score of 4 and 5) converge on PI3K/mTOR signaling pathway. PI3K inhibitors AS605240 (110 nM), Wortmannin (230 nM), LY-294,002 (15 μM), and mTOR inhibitors Temsirolimus (30 μM), and PP242 (15 μM) all fully rescue the bace2−/− hyperdendritic melanophores.
(D) Quantification of tailfin melanophore cell area at 72 hpf with hits from the screen (n = each fish, one-way ANOVA followed by Holm-Sidak's multiple comparisons test, ∗∗∗∗p < 0.0001).
(E–G) Morpholinos knockdown of the PI3K γ isoform and mTOR in bace2−/− mutants rescue the phenotype analogous to what is seen with compounds from the screen, and the resulting tailfin melanophore cell area is quantified in (F) and (G). The data are from three independent experiments. Uninjected bace2−/− siblings (Control) are scored in the same manner, two-tailed t test, ∗∗∗∗p < 0.0001. All bar graphs are presented as means ± SEM. Scale bar, 100 μm.
See also Figures S3–S5.
Reprinted from Developmental Cell, 45(5), Zhang, Y.M., Zimmer, M.A., Guardia, T., Callahan, S.J., Mondal, C., Di Martino, J., Takagi, T., Fennell, M., Garippa, R., Campbell, N.R., Bravo-Cordero, J.J., White, R.M., Distant Insulin Signaling Regulates Vertebrate Pigmentation through the Sheddase Bace2, 580-594.e7, Copyright (2018) with permission from Elsevier. Full text @ Dev. Cell