Fig. 6
Nitric Oxide Is Necessary for Early Demyelination
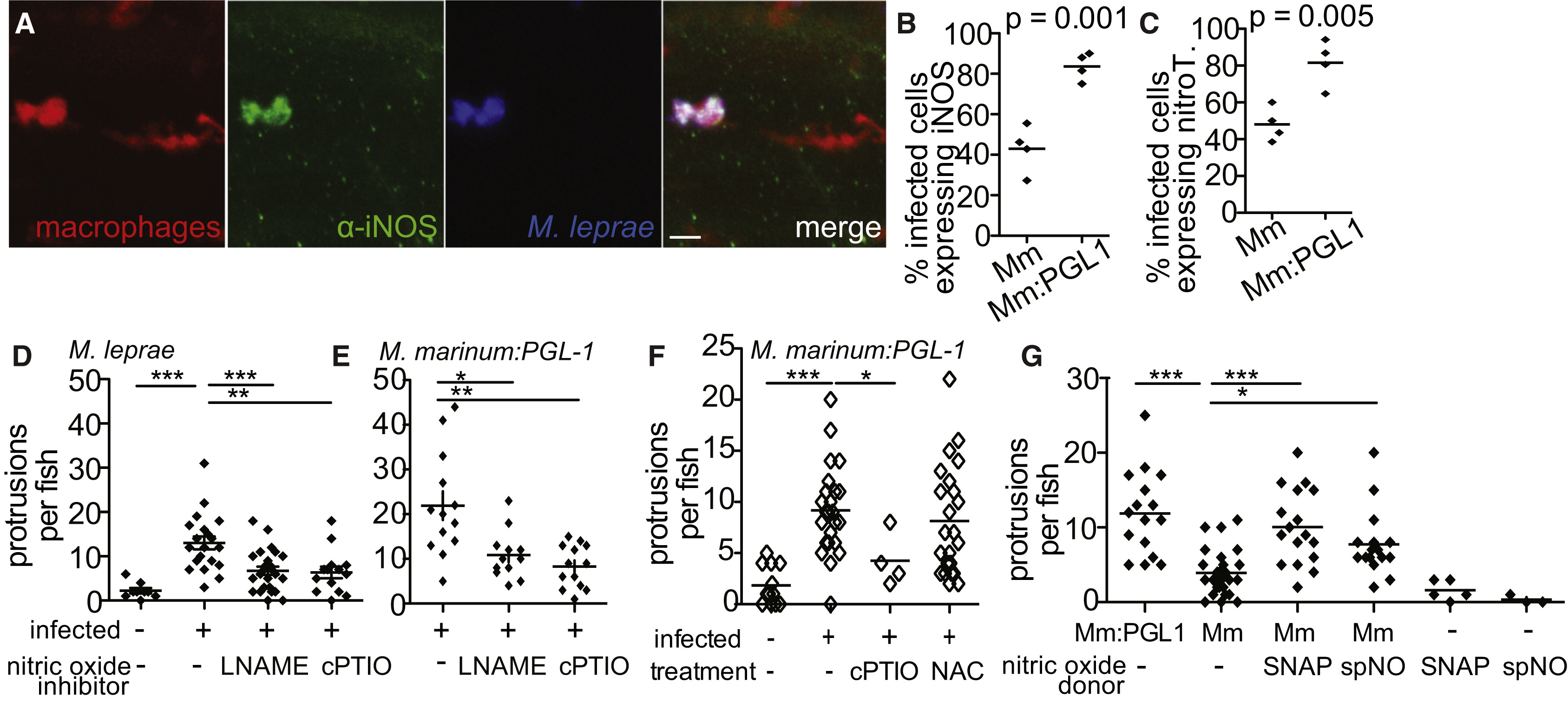
(A) Representative confocal images of a macrophage aggregate in the spinal cord of an mpeg1 larva infected with M. leprae and stained with α-iNOS antibody. Scale bar, 10 μm.
(B) Mean percentage of infected mpeg1-positive macrophages that also express iNOS in 7 dpf larvae 5 dpi with WT M. marinum or M. marinum:PGL-1. (Student’s t test.)
(C) Mean percentage of infected mpeg1-positive macrophages that stain with α-nitrotyrosine antibody (nitroT) in larvae like in (B). (Student’s t test).
(D) Mean number of myelin protrusions per animal in 5 dpf mbp larvae 2 dpi with M. leprae, which were treated with 0.5% DMSO vehicle (-), iNOS inhibitor (L-NAME), or ROS/RNS scavenger (cPTIO). (∗∗p < 0.01; ∗∗∗p < 0.001; one-way ANOVA with Dunnett’s multiple comparison test.)
(E) Mean number of myelin protrusions per animal in 5 dpf mbp larvae 2 dpi with M. marinum:PGL-1, treated like in (D). (∗p < 0.05; ∗∗p < 0.01; one-way ANOVA with Dunnett’s multiple comparison test.)
(F) Mean number of myelin protrusions per animal in 5 dpf mbp larvae 2 dpi with M. marinum:PGL-1, which were treated with 0.5% DMSO vehicle (“-”), nitric oxide scavenger (cPTIO), or ROS scavenger NAC. (∗p<0.05; ∗∗∗p<0.001; one-way ANOVA with Dunnett’s multiple comparison test.)
(G) Mean number of myelin protrusions per animal in larvae infected like in (F), which were soaked post-injection in 0.5% DMSO vehicle (“-”) or in nitric oxide donors SNAP or spermine NONOate (spNO). (∗p<0.05; ∗∗∗p<0.001; one-way ANOVA with Dunnett’s multiple comparison test.)
See also Figure S5.