Fig. 2
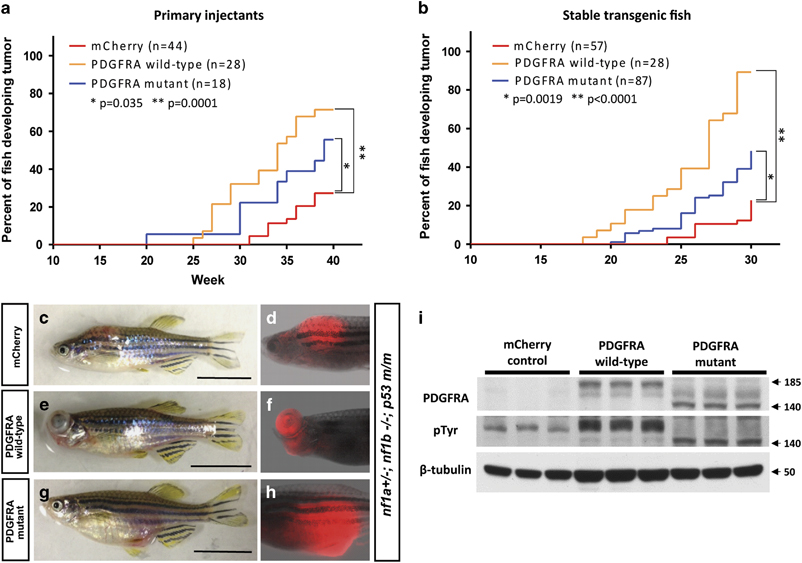
Both wild-type and constitutively activated mutant forms of PDGFRA accelerate MPNST tumorigenicity in nf1- and p53-deficient fish. (a and b) Kaplan–Meier analysis of tumorigenesis in fish with either mosaic or stable expression of the PDGFRA transgene. Onset of MPNSTs in nf1- and p53-deficient zebrafish (nf1a+/–; nf1b–/–; p53m/m) injected with the following DNA constructs: (1) sox10:mCherry alone (mCherry, red); (2) sox10:PDGFRA wild-type and sox10:mCherry (PDGFRA wild-type, yellow); or (3) sox10:PDGFRA mutant and sox10:mCherry (PDGFRA mutant, blue). Note that wild-type PDGFRA overexpression accelerated the onset of MPNSTs more rapidly than constitutively activated mutant PDGFRA. (c–h) Representative images of the sox10 promoter driving mCherry-positive tumors under different conditions. Transgenic sox10:mCherry (c and d), sox10:mcherry/PDGFRA wild-type (e and f) and sox10:mCherry/PDGFRA CA mutant (g and h) in the nf1a+/−; nf1b−/−; p53m/m background induced tumors that strongly expressed mCherry protein (>30 w.p.f., scale bar=10 mm). (i) Western blot analysis for PDGFRA in protein lysates prepared from tumors of mCherry control, PDGFRA wild-type and PDGFRA mutant fish. PDGFRA wild-type, mutant and their phosphorylated (active) forms were detected. β-Tubulin was used an internal control for equal loading. Arrow denotes protein size (kDa).