Fig. 4
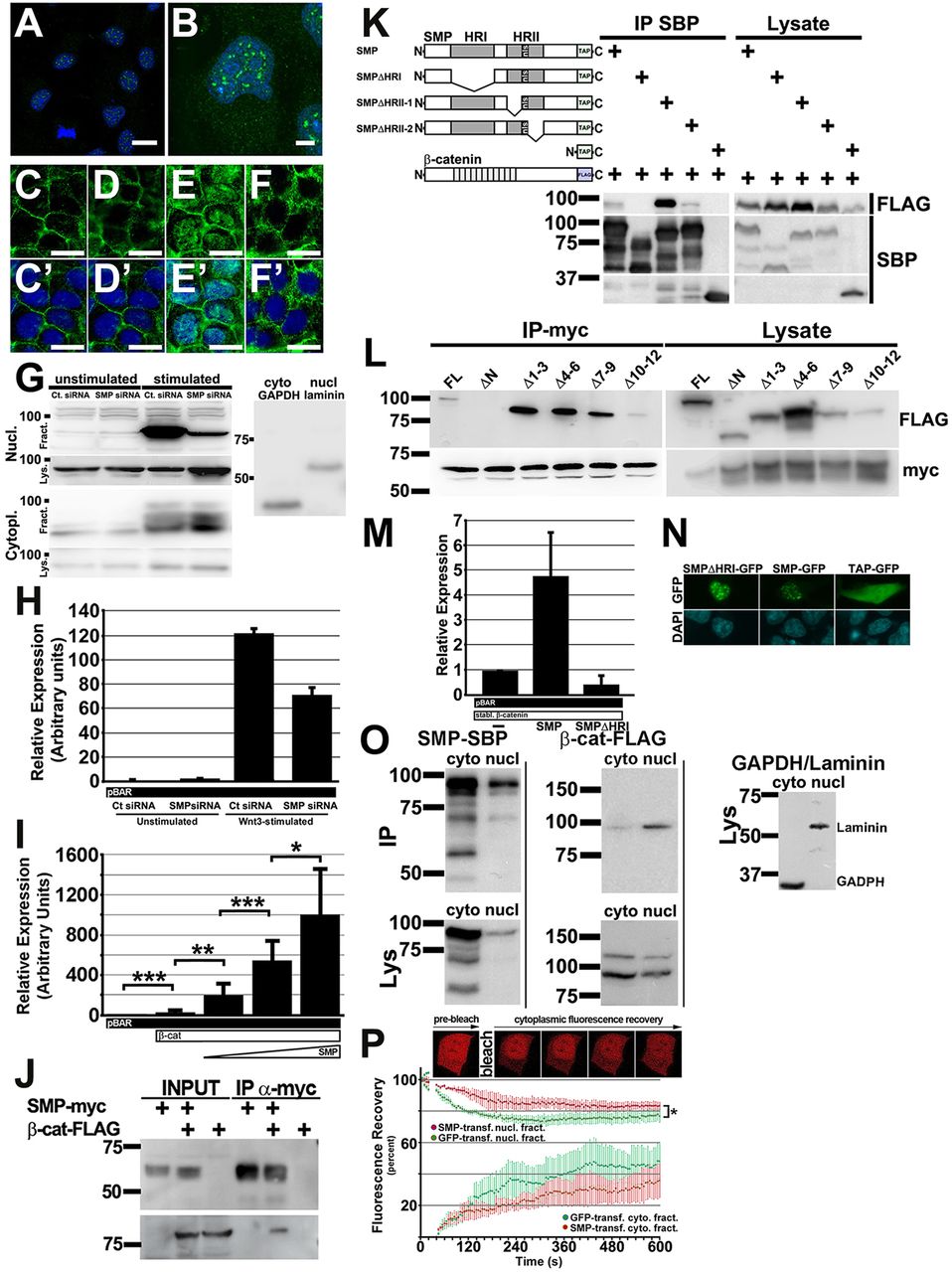
Mammalian SMP (FAM53B) regulates β-catenin similarly to zebrafish Smp and interacts with β-catenin to retain it in the nucleus. (A) Immunocytochemistry (ICC) for the human SMP (green) in the Hoechst-stained nuclei (blue) of HEK293T cells. (B) Higher magnification shows the protein localizes as foci in Hoechst-stained nuclei. (C-F) β-Catenin ICC (green) of control siRNA-transfected unstimulated HEK293T cells (C), SMP siRNA-transfected HEK293T cells (D). control siRNA-transfected HEK293T cells stimulated with Wnt3-conditioned medium (E) and SMP siRNA-transfected HEK293T cells stimulated with Wnt3-conditioned medium (F). (C′-F′) β-Catenin ICC merged with DAPI (blue). (G) Representative subcellular fractionation blots showing the protein levels of nuclear (Nucl.) and cytoplasmic (Cytopl.) β-Catenin in unstimulated and Wnt3-stimulated HEK293T cells transfected either with control (Ctr) siRNA or Smp siRNA. Fract. denotes nuclear or cytoplasmic fractionation blots. Lys. denotes blots for loading control of total lysates for each fraction. Laminin and GAPDH show clear separation of the fractions. The numbers indicate protein ladder positions in kDa. (H) Fold activation of pBAR luciferase reporter from assays of control and Smp siRNA knockdown HEK293T cells without and with Wnt3-condition medium stimulation (Wnt). P<0.01 between Ct and SMP siRNA Wnt3-stimulated groups. Data are the average±s.d. (I) Dose-dependent activation of β-catenin-responsive promoter by human SMP. *P<0.05, **P<0.001, ***P<0.008. Data are the average±s.d. (J) Immunoprecipitation blot using anti-Myc antibody to pull down Myc-tagged human SMP shows co-immunoprecipitation of Flag-tagged human β-catenin as detected by anti-Flag antibody. (K) Immunoprecipitation experiment using different TAP-Tagged (TAP) SMP deletion mutants to determine which conserved domain interacts with FLAG-tagged β-catenin. (L) Immunoprecipitation experiment of SMP-myc for different FLAG-tagged β-catenin deletion mutants lacking either the N terminus or different sets of armadillo repeats. The lysate gels show the level of expression of each construct. (M) Luciferase assay of HEK293T cells transfected with: pBAR and stabilized β-catenin; pBAR, stabilized β-catenin and full-length SMP; or pBAR, stabilized β-catenin and SMP lacking the first homology domain (SMP-ΔHRI). P<0.01 between all groups. Data are the average±s.d. (N) Transfection of HEK293T cells with TAP-tagged GFP or SMP-GFP or SMP-ΔHRI-GFP shows nuclear localization of both SMP expression constructs. (O) Blot of subcellular fractionation (nuclear versus cytoplasmic) experiment for transfected TAP-tagged Smp and FLAG-tagged β-catenin. Immunoprecipitation blots (IP) are above. Lysate blots (Lys) are below. Lysate blot probed with GAPDH and laminin shows clear separation of each fraction. (P) β-Catenin-mCherry fluorescence before bleaching and at increasing times after photobleaching the cytoplasm of HEK293T cells co-transfected either with GFP or SMP-GFP. The graph shows the decrease in nuclear β-catenin-mCherry and its cytoplasmic recovery. *P<0.05. Scale bars: 20μm in A,B; 5μm in C-F2′. Data represent at least three or more independent experiments.