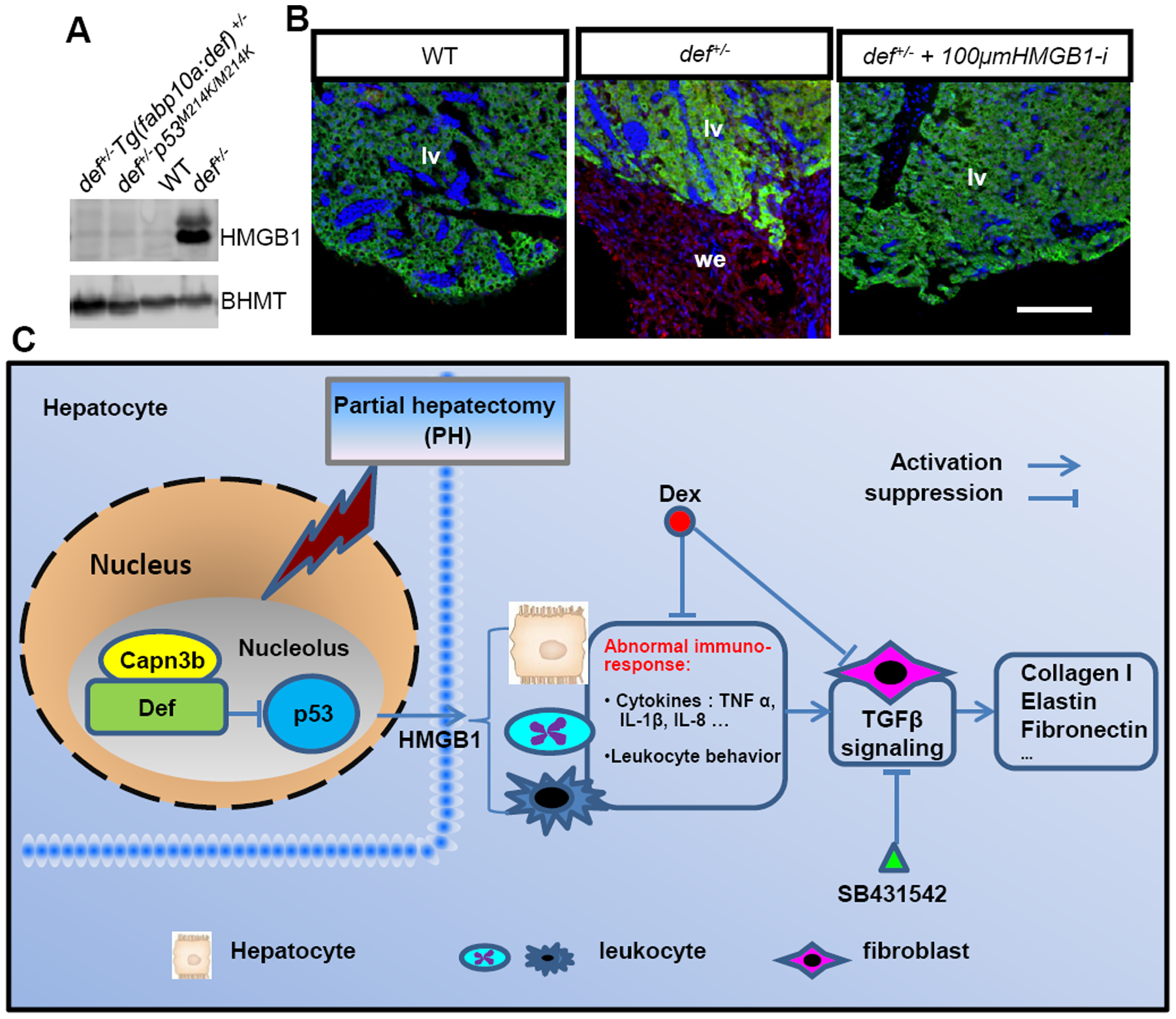
Fig. 11
A model to explain how Def haploinsufficiency activates the p53-dependent inflammation-mediated TGFβ signalling and causes fibrotic scar formation in def+/- after PH.
(A) Western blot of HMGB1 in the adult liver from wild-type, def+/-, def+/-Tg(fabp10:def)+/- and def+/-p53M214K/M214K fish. Loading control: Bhmt. (B) Immunostaining of ColI and Bhmt in the wound epidermis in wild-type, def+/- and def+/-fish treated with 100 μM of glycyrrhizic acid ammonium 5 days after PH. lv, liver; we, wound epidermis. (C) In a wild-type liver, Def complexes with Capn3 to mediate p53 degradation in the nucleoli to mitigate the inflammatory response after acute injury. In def+/- fish, the activity of the Def-Capn3 protein degradation pathway is compromised and p53 protein is thus stabilised, which in turn activates the p53 pathway. Constant activation of the p53 pathway up-regulates the expression of the pro-inflammatory factor HMGB1 that up-regulates the expression of cytokines (e.g., TNFα, IL-1β, IL-6 and IL-8) and other signalling pathways that lead to a prolonged inflammatory response in def+/-. The prolonged inflammatory response activates TGFβ signalling and causes the over-production of fibrotic molecules (e.g., collagens, elastin and fibronectin) in the wound epidermis that finally forms a fibrotic scar at the amputation site in def+/-. Fibrotic scar formation in def+/- can be blocked by over-expressing Def specifically in the hepatocytes, by the loss of function of p53, and by treatment with Dex (anti-inflammatory drug), SB431542 (inhibitor of TGFβ signalling) or glycyrrhizic acid ammonium (inhibitor of HMGB1 function).