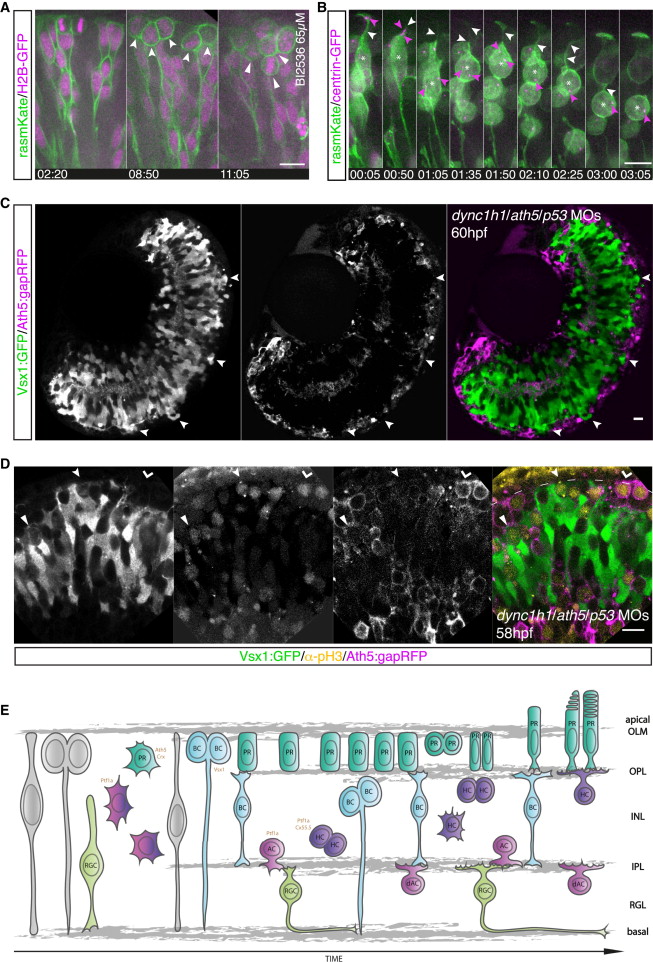
Fig. 4
Correlation between Apical Crowding and Repositioning of Mitotic Cells to Nonapical Locations
(A) The Plk1 inhibitor BI2536 leads to mitotic arrest. Embryos are mosaically injected with rasmKate (green) and H2B-GFP (magenta). Neuroepithelial progenitors translocate their nuclei to the apical side and undergo apical mitosis (left). Cells enter mitosis at the apical side but fail to undergo division, leading to apical accumulation of mitotic cells (notched arrow, middle). Emergence of additional layers of mitotic cells at subapical locations (filled arrow, right); from Movie S6. Time in hr:min. Imaging started at 32 hpf.
(B) Embryos were mosaically injected with rasmKate (green) and centrin-GFP (magenta) and treated with BI2536 (65 μM). A retinal progenitor cannot reach the apical side and enters mitosis subapically (asterisk) upon apical congestion. The apical location of the centrosome is shown (00:05, pink arrows). The centrosome moves toward the nucleus (01:35). The apical process is present at the onset of cell rounding (01:35, white arrows) but lost after centrosome migration (03:05). Time in hr:min. Imaging started at 36 hpf.
(C) Discontinuous PR layer formation in Ath5:gapRFP (magenta) dync1h1/ath5/p53 morphants (MO). In PR-free areas, Vsx1:GFP (green) cells reach the apical side (arrows).
(D) pH3 staining (yellow) in Vsx1:GFP/Ath5:gapRFP (green/magenta) dync1h1/ath5/p53 morphant embryos. Vsx1+ divisions are observed subapically in regions with an Ath5+ PR layer (filled arrow) but apically in regions in which the PR layer is disrupted (notched arrow). Apical Ath5+ divisions are observed in regions in which an Ath5+ cell layer forms (kinked arrow).
(E) Model of zebrafish retinogenesis, taking previous data and our findings into account.
Scale bars represent 10 μm. See also Figure S3.