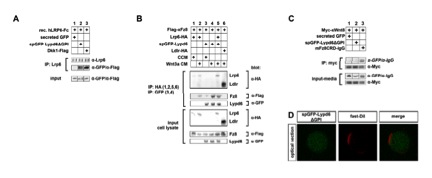
Fig. S4
Lypd6 directly interacts with Lrp6, while it binds to Fz8 in a Wnt3a-dependent manner but does not interact with Wnt8.
related to Figure 5.
(A) Purified secreted Lypd6 (spGFP-Lypd6ΔGPI) co-IPs with secreted recombinant hLrp6-Fc chimeric protein (lane 2), while secreted GFP does not (lane 1). Dkk1-Flag can also be co-IPed with hLrp6-Fc (lane 3).
(B) Flag-tagged Xenopus Frizzled8 (xFz8) co-IPs with spGFP-Lypd6 in HEK293T cells and this interaction is strongly enhanced by Wnt3a (compare lanes 3 & 4). Flag-xFz8 can also be IPed with Lrp6- HA in the presence of Wnt3a, but not with Ldlr-HA. Lypd6 and Lrp6 still bind to Fz8 when expressed together.
(C) Immunoprecipitation experiments of conditioned media show that Myc-tagged Xenopus Wnt8 (MycxWnt8) does not bind to spGFP-Lypd6ΔGPI or the negative control secreted GFP, while it does bind to the positive control, the CRD domain of mouse Fz8 fused to IgG (mFz8CRD-IgG).
(D) spGFP-Lypd6ΔGPI does not localize to the membrane of GPMVs derived from CHO cells.
Reprinted from Developmental Cell, 26(4), Özhan, G., Sezgin, E., Wehner, D., Pfister, A.S., Kühl, S.J., Kagermeier-Schenk, B., Kühl, M., Schwille, P., and Weidinger, G., Lypd6 Enhances Wnt/beta-Catenin Signaling by Promoting Lrp6 Phosphorylation in Raft Plasma Membrane Domains, 331-345, Copyright (2013) with permission from Elsevier. Full text @ Dev. Cell