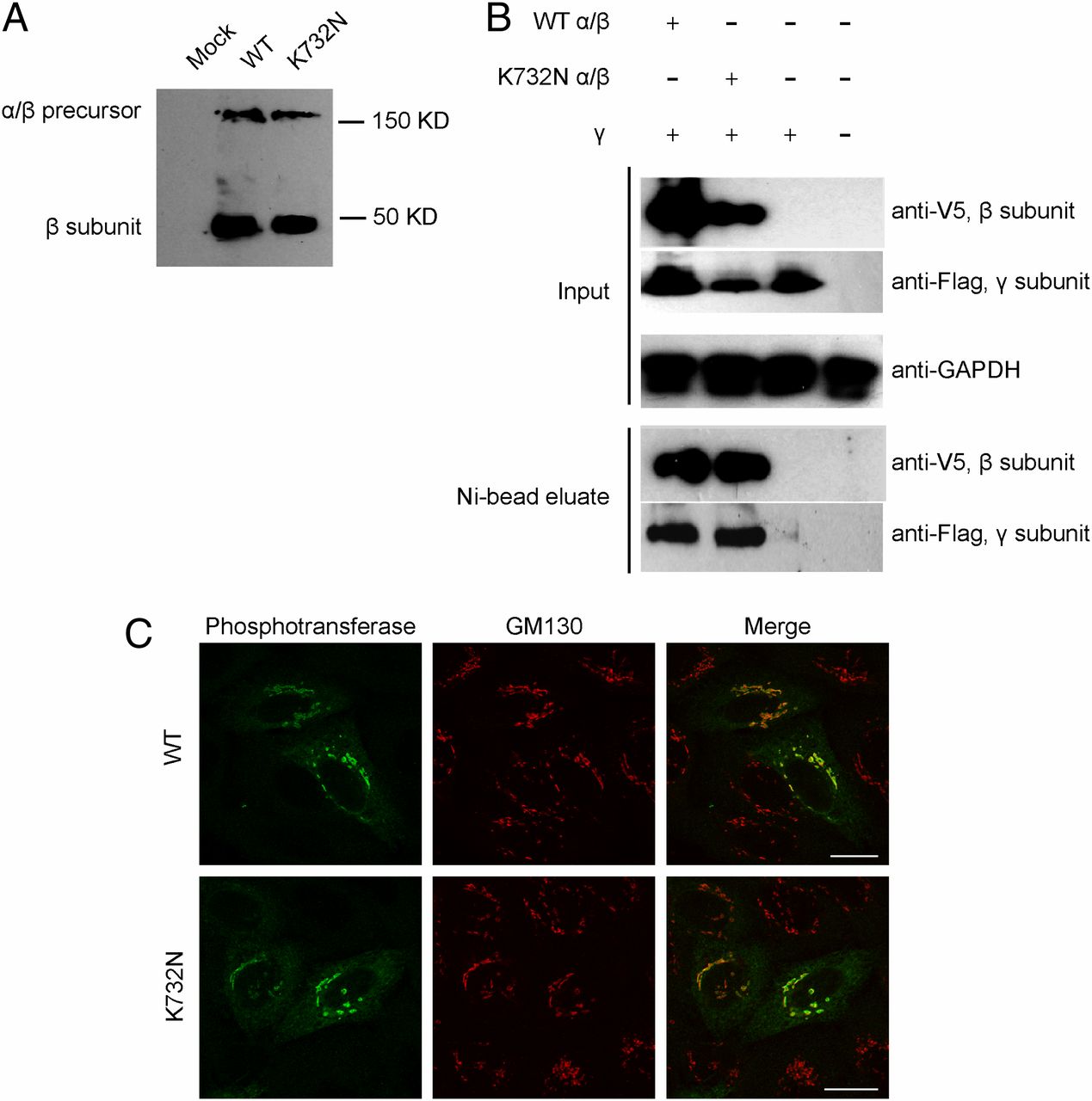
Fig. 2
The K732N mutant exhibits normal expression, interaction with the γ subunit, processing, and localization. (A) HEK293 cells were transfected with plasmids encoding WT or K732N mutant α/β cDNAs with a C-terminal His/V5 tag. After 48 h, cell extracts were prepared and subjected to SDS/PAGE and immunoblotting with anti-V5 antibody to detect the α/β precursor and the cleaved β subunit. (B) Lysates of HEK293 cells expressing WT or K732N mutant α/β subunits with His/V5 tags plus γ subunits with a Flag tag or γ subunit alone were incubated with Ni-NTA agarose to bind the β subunit. Bound proteins were eluted and subjected to SDS/PAGE and immunoblotting with anti-V5 to detect the β subunit and anti-Flag to detect the γ subunit. (Upper) The expression of proteins in the original lysates (Input), with GAPDH serving as a loading control. (Lower) The proteins bound and eluted from the Ni-NTA resin (Ni-bead eluate). Nontransfected cells served as an additional control. (C) HeLa cells were transfected with WT (Upper) or K732N (Lower) α/β cDNA in pcDNA6 and fixed with 4% paraformaldehyde/PBS 16 h after transfection. The cells were stained for immunofluorescence microscopy with antibodies to the alpha subunit of phosphotransferase (green) and the cis-Golgi marker GM130 (red). (Scale bars, 20 μm.)