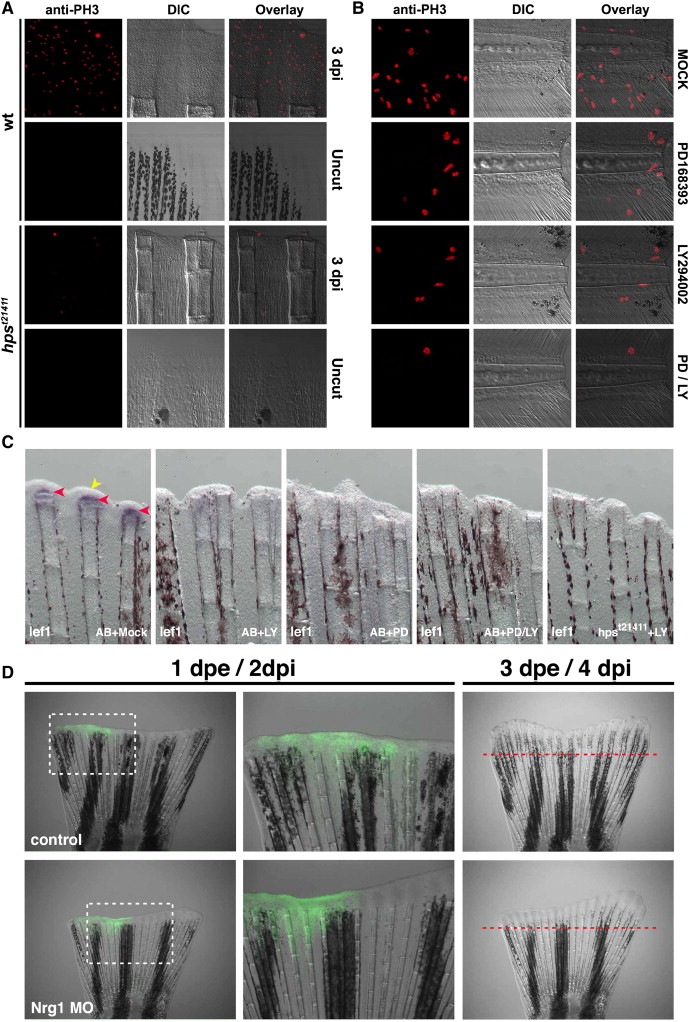
Fig. 8 PI3K functionally interacts with ErbB2 and ErbB3 during regeneration. (A) Adult homozygous mutants for hpst21411 have a significantly reduced amputation-induced progenitor proliferation measured after 3 dpi compared to wild-type controls (Fig. S6). Similarly, amputation-induced progenitor proliferation in the adult caudal fin at 1 dpi is reduced upon incubation with 5 μM of the PI3K inhibitor LY294002 or 10 μM of the ErbBs inhibitor PD168393 for 24h. Importantly, upon simultaneous incubation with both inhibitors the amount of proliferating cells is reduced even more than in fins incubated with either alone (data not shown). (B) Similar to the adult situation, the simultaneous incubation with the and ErbB inhibitors significantly diminished the number of proliferating cells in the region A + B of the regenerate compared to amputated larvae incubated with either inhibitor alone or in DMSO. This trend is observed all over the duration of the regeneration response (Fig. S6). (C) ErbB and PI3K signaling interact to modulate regeneration in zebrafish. Images of amputated caudal fins at 24 hpi in wild-type or hpst21411 ErbB3 homozygous mutants. Normally, lef1 is transcribed in the basal layer of the regenerating epidermis at 24 hpi (red arrowheads). The wounded epithelium is also clearly visible at this stage (yellow arrowhead). Animals incubated with 10 μM of the PI3K inhibitor LY294002 exhibit an evident decreased on lef1 expression. Incubation with 10 μM PD168393 show a phenotype similar to that observed with LY294002. The thickness of the wounded epithelium is markedly reduced upon incubation with 10 μM LY294002 and 10 μM PD168393. Similarly, homozygous mutants for the ErbB3 allele hpst21411 incubated with 10 μM LY294002 lack any detectable lef1 expression or wound epithelium. (D) Electroporation of a morpholino designed against NRG1 disturbs regeneration while a control morpholino designed against rx3 does not. The difference in length of the regenerates that grew within 3 days post electroporation (dpe) between the two treatments was significant (98% ± 5.3% for the control morpholino and 54.2% ± 7.1% for the Nrg1 MO, P <0.005). The data depicts the percent length of the regenerate of the experimental side in relation to the control side (e.g. the other half of the same fin).
Reprinted from Developmental Biology, 327(1), Rojas-Muñoz, A., Rajadhyksha, S., Gilmour, D., van Bebber, F., Antos, C., Rodríguez Esteban, C., Nüsslein-Volhard, C., and Izpisúa Belmonte, J.C., ErbB2 and ErbB3 regulate amputation-induced proliferation and migration during vertebrate regeneration, 177-190, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.