For practical purposes, i.e. evaluation of effects of toxicants, a simplified classification, e.g. distinguishing between previtellogenic and vitellogenic oocytes, may be sufficient. Alternatively, histomorphometrical quantification of oocyte sizes (surface area, diameter) may be helpful to analyse toxicological effects.
All images below are taken from adult female zebrafish ovaries; H&E or PAS staining. Some sets show clusters of cells with a single cell highlighted.
oogonium
- develops in clusters from germinative epithelium; cell diameters range from 10-20 µm;
- large nucleus with one centrally located nucleolus.
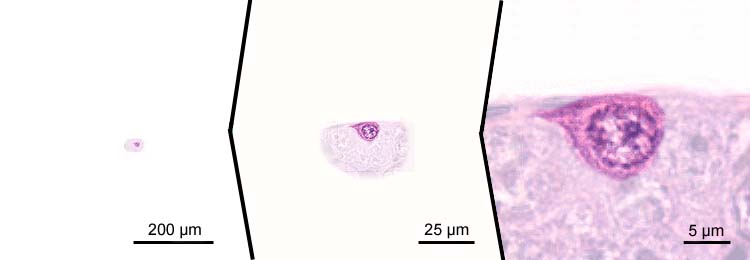
proliferating oogonia
- small cells, cell diameters less than 10 µm;
- multiplication of oogonia provides a continuous supply of cells to replace oogonia developing to oocytes;
- threadlike chromosomes;
- cells lie in nests: pre-follicle phase of primary growth.

primary oocytes - stage 1; first meiotic division - leptotene stage
- part of the stock of oogonia develops into primary oocytes, which enter the first meiotic division;
- cell diameters range from 10-20 µm;
- meiosis starts with duplication of the chromosomes, yielding a tetraploid cell; the resulting sister chromatides remain attached to the centromere;
- in the initial stage of the first meiotic division, the leptotene stage, chromosomes condense from their interphase conformation to produce thin, threadlike structures, seen randomly distributed throughout the whole nucleus; both ends of each chromosome attach to the nuclear envelope: "chromatin-nucleolus" phase;
- cells lie in nests: pre-follicle phase of primary growth.

primary oocytes - stage 1; first meiotic division - zygotene stage
- part of the stock of oogonia develops into primary oocytes, which enter the first meiotic division;
- cell diameters range from 10-20 µm;
- the zygotene stage starts with the joining of homologous chromosomes (synapsis); the resulting complexes of chromosome pairs are known as bivalents or tetrads (two pairs of sister chromatids);
- this process shows as condensations in the nucleus: "chromatin-nucleolus" phase;
- cells lie in nests: pre-follicle phase of primary growth.

primary oocytes - stage 1; first meiotic division - zygotene stage
- cell diameters range from 10-20 µm;
- the chromosomes assemble at one pole of the nucleus, opposite to the nucleolus: "chromatin-nucleolus" phase;
- cells lie in nests: pre-follicle phase of primary growth.

primary oocytes - stage 1; first meiotic division - pachytene stage
- cell diameters about 20 µm;
- in the pachytene stage, appearing recombination nodules mediate chromosomal exchange, resulting in nonsister chromatid crossovers (i.e. between chromatids each from the other of the paired chromosomes);
- the chromosomes take a thickened appearance: "chromatin-nucleolus phase";
- increase of size of the nucleus;
- cells lie in nests: pre-follicle phase of primary growth.

primary oocytes - stage 1; first meiotic division - pachytene stage
- cell diameters about 20 µm;
- appearance of many small nucleoli throughout the nucleus;
- chromosomes spread out through the nucleus;
- increase of size of the nucleus;
- cells "leave" nests: transition from pre-follicle to follicle phase of primary growth.
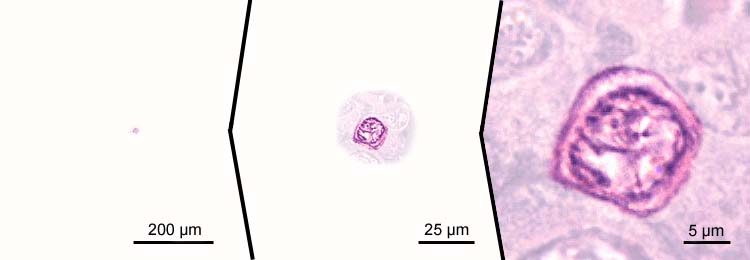
primary oocytes - stage 1; first meiotic division - diplotene stage
- increase of cell size, cell diameters > 20 µm;
- increasing amount of cytoplasm, which becomes basophilic
- the diplotene stage is characterized by desynapsis (homologous chromosomes move away from each other); decondensed chromosomes engage in RNA synthesis for production of oocyte storage material;
- chromosomes have a slender appearance;
- nucleoli move to the periphery of the nucleus: "perinucleolus" stage;
- increase of size of the nucleus, which is further referred to as "germinal vesicle";
- appearance of accompanying follicle cells: follicle phase of primary growth.

primary oocytes - stage 2 (previtellogenic)
- increase of cell size, cell diameters 20-90 µm;
- increase of size of the germinal vesicle;
- "perinucleolus" stage;
- follicle cells are present, follicle phase of primary growth.

primary oocytes - stage 2 (previtellogenic)
- cell diameters 20-90 µm;
- round-oval germinal vesicle with 1-4 nucleoli per section;
- "perinucleolus" stage, follicle phase of primary growth;
- follicle cells are arranged in a single layer.

primary oocytes - stage 3 (previtellogenic)
- further increase of cytoplasm volume with corresponding increase in cell diameters to a range of 80-160 µm;
- the cytoplasm still reacts basophilic but shows spotted clearing;
- "perinucleolus" stage, follicle phase of primary growth;
- initial development of the zona radiata;
- accompanying follicle cells differentiate to a granulosa layer and a theca layer.

primary oocytes - stage 4
- further increase of cytoplasm volume with corresponding increase in cell diameters up to 140-270 µm;
- initial endogenous vitellogenesis, visible as small chromophobic or slightly eosinophilic vacuoles in a zone in the cytoplasm, halfway the nucleus and the cell membrane; such yolk vesicles or intravesicular yolk is strongly PAS positive;
- "oil droplet phase", "cortical alveolus stage";
- the zona radiata is clearly visible (thickness ~2 µm);
- accompanying follicle cells are present.

primary oocytes - stage 4
- further increase of cytoplasm volume with corresponding increase in cell diameters up to 270 µm;
- ongoing endogenous vitellogenesis, with larger chromophobic vacuoles (PAS positive) in a central zone in the cytoplasm
- "oil droplet phase", "cortical alveolus stage";
- the zona radiata is clearly visible (thickness ~2 µm);
- growth of the granulosa layer, which originates from the follicle cell layer (thickness ~4 µm).

primary oocytes - stage 5
- expansion of endogenous vitellogenesis to the periphery of the cytoplasm;
- increase of the cell diameter to 300 µm;
- "oil droplet phase", "cortical alveolus stage";;
- increase of thickness of the zona radiata (5 µm);
- granulosa layer thickness ~4 µm.

primary oocytes - stage 5
- expansion of endogenous vitellogenesis to the entire cytoplasm;
- increase of the cell diameter to 400 µm;
- initiation of exogenous vitellogenesis, visible as eosinophilic granula; this constitutes extravesicular yolk or yolk globules and is only weakly positive to PAS;
- vitellogenesis stage, yolk stage;
- increase of thickness of the zona radiata (5 µm); note its radiant appearance;
- granulosa layer thickness ~4 µm.
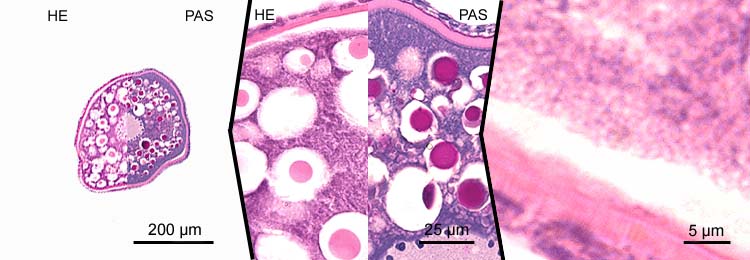
primary oocytes - stage 6
- marked vitellogenesis with corresponding increase of the cell diameter to ~400 µm;
- vitellogenesis stage, yolk stage, growth phase;
- shift of extravesicular (exogenous) granula to the perinuclear region;
- concentration of endogenous vitellogenin vacuoles in the periphery of the cytoplasm;
- increase of thickness of the zona radiata (5-10 µm);
- thickness of the granulosa layer is variable (~2-6 µm).
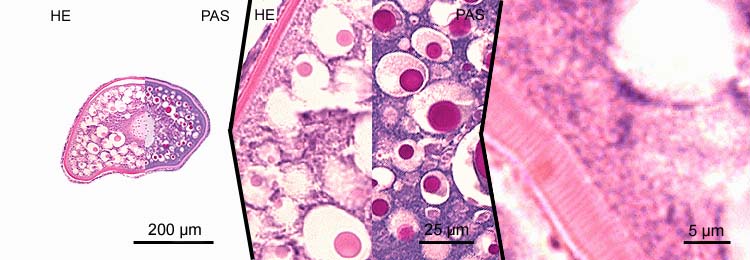
primary oocytes - stage 6-7
- marked vitellogenesis with corresponding increase of the cell diameter to ~600 µm;
- vitellogenesis stage, yolk stage, growth phase;
- shift of extravesicular (exogenous) granula to the perinuclear region and fusion of extravesicular (exogenous) granula;
- concentration of endogenous vitellogenin vacuoles to a small band in the periphery of the cytoplasm;
- increase of thickness of the zona radiata (~10 µm);
- thickness of the granulosa layer is variable (~2-6 µm).

primary oocytes - stage 6-7
- marked vitellogenesis with corresponding increase of the cell diameter to ~800 µm;
- vitellogenesis stage, yolk stage, growth phase;
- shift of extravesicular (exogenous) granula to the perinuclear region and fusion of extravesicular (exogenous) granula;
- concentration of endogenous vitellogenin vacuoles to a small band in the periphery of the cytoplasm;
- increase of thickness of the zona radiata (~10 µm);
- thickness of the granulosa layer is variable (~2-6 µm).
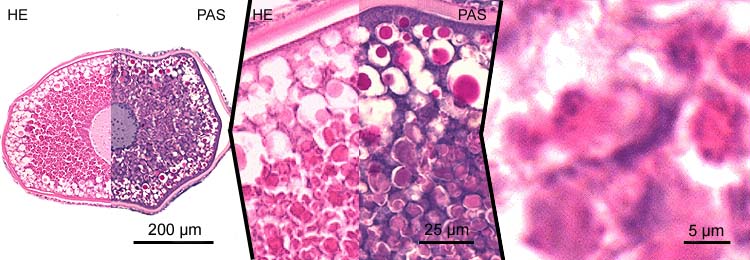
secundary oocytes - stage 7
- vitellogenesis stage, yolk stage, growth phase;
- at the end of the growth phase, the germinal vesicle moves to the animal pole of the cell. At this region, there is a minute, funnel-shaped perforation in the chorion, the so-called micropyle, which serves as the future entrance of the sperm. This micropyle is the result of inhibition of membrane formation by a specialised follicle cell, the micropylar cell (see image), which has an intimate relation with the oocyte 3.
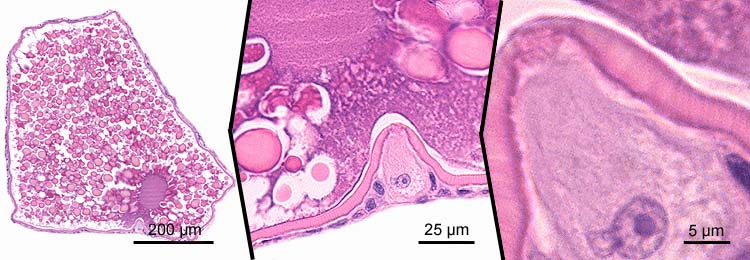
secundary oocytes - stage 7
- maturation stage
- at the end of the growth phase, the first meiotic division is completed to produce a large diploid secundary oocyte and a small diploid polar body (not shown); subsequently, the second meiotic division is initiated (not shown) and an oocyte with the following characteristics is ovulated:
- marked vitellogenesis with corresponding increase of the cell diameter to ~600 µm;
- fusion of extravesicular (exogenous) granula
- no distinct endogenous vitellogenin vacuoles
- decrease of thickness of the zona radiata (~2-5 µm)
- decrease of thickness of the granulosa layer (~2-5 µm);
- the second meiotic division is completed after fertilization
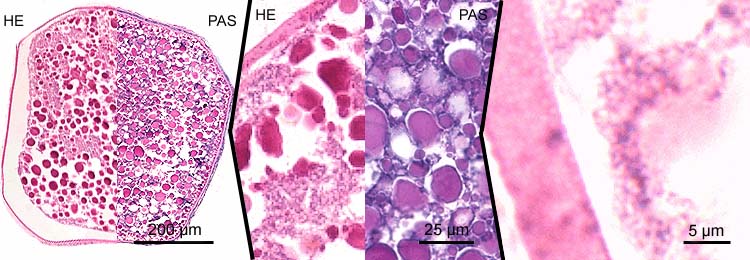
follicle derivatives - corpus atreticum (preovulatory)
- composed of hypertrophic granulosa cells and oocyte remnants: cell and membrane debris (the brown pigment is lipofuchsin, the end product of lysosomal digestion); the shown corpus atreticum also contains resistant zona radiata remnants, which are quickly digested in another type corpus atreticum.

follicle derivatives - corpus atreticum (postovulatory)
- shows as a collapsed envelope of former granulosa cells;
- hypertrophic cells with lobulated nuclei;
- short-lived: the structure starts to decline within a day, and will be cleared away within seven days.

References
- Lambert-JG. The ovary of the guppy Poecilia reticulata. The granulosa cells as sites of steroid biosynthesis. Gen. Comp Endocrinol. 15:464-476; 1970.
- Selman-K, Wallace-RA, Sarka A, Qi-X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J. Morphol. 218:203-224; 1993.
- Yamamoto-TS. Morphological and cytochemical studies on the oogenesis of the fresh water fish, medaka (Oryzias latipes) (in Japanese, referred at The medaka fish homepage: stages in development of medaka). Japan J. Ichthyol. 4: 170-181; 1955.