- Title
-
The zebrafish foxj1a transcription factor regulates cilia function in response to injury and epithelial stretch
- Authors
- Hellman, N.E., Liu, Y., Merkel, E., Austin, C., Le Corre, S., Beier, D.R., Sun, Z., Sharma, N., Yoder, B.K., and Drummond, I.A.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
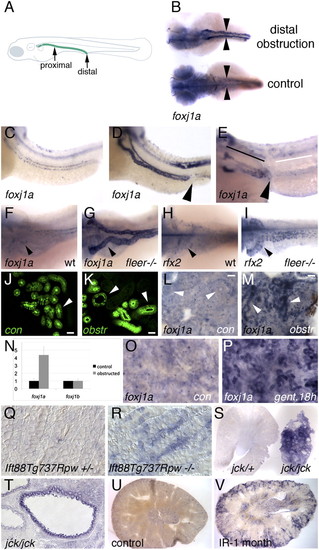
Up-regulation of foxj1a expression in epithelial injury and cystic distension. Embryos in B-I were all imaged at 56 hpf. (A) Schematic diagram illustrating location of the zebrafish pronephric duct (green line) and sites of mechanical proximal and distal obstruction. (B) foxj1a is strongly up-regulated in pronephros of distally obstructed embryos (top), whereas baseline expression is low in paired pronephric ducts (arrowheads) of unobstructed control embryos (bottom). Dorsal views of embryos are shown. Treatment with cycloheximide does not prevent obstruction-mediated increase in foxj1a expression (D) compared with unobstructed embryos (C). (E) Proximal pronephric obstruction (arrowhead) results in up-regulation of foxj1a only in dilated regions proximal to site of obstruction (black line) and not in tubule cells distal to obstruction (white line). (F–I) Both foxj1a and rfx2 are up-regulated in zebrafish cystic kidney mutants. (F) In WT (wt) embryos, foxj1a was expressed at low level in pronephric epithelial cells. (G) foxj1a expression was strongly up-regulated in dilated pronephric tubules of fleer mutant tubules, which develop cysts. Similarly, rfx2 gene expression was up-regulated in dilated kidney tubules of fleer mutants (I) compared with controls (H). (J-M) Obstruction-mediated induction of foxj1a expression in adult zebrafish kidney. Distal mesonephric obstruction in adult zebrafish results in tubule dilation after 12 h (K) compared with control sham-operated kidneys (J) visualized using the Tg(atase1a1a.4:eGFP) transgenic line (30). Whole-mount in situ hybridization of adult zebrafish kidney with foxj1a probe shows low level of expression in sham-operated control (L), whereas distal obstruction (M) induces foxj1a expression in mesonephric tubules after 18 h (arrowheads). (Scale bar: N-Q, 25 μm.) (N) Quantification of foxj1a and foxj1b expression by quantitative RT-PCR in obstructed embryos. (O) foxj1a is expressed at low level in whole-mount adult zebrafish kidney, whereas foxj1a is up-regulated in tubule cells after 18 h of treatment with the nephrotoxin gentamicin (P). (Q) Sections of WT post-embryonic day-7 mouse kidneys stained for Foxj1 mRNA expression show low level of Foxj1 RNA, whereas similar sections of Ift88Tg737Rpw-/- mutant mouse kidneys (R) show strong cortical expression of Foxj1 in subset of tubule cells. (S) jck/+ Kidney slice (left) compared with slice of cystic jck/jck kidney (right), revealed strong up-regulation of Foxj1 in jck/jck cystic kidney. (T) Section of jck/jck kidney showing strong expression in cyst lining epithelial cells. Increased Foxj1 expression was also observed in mice subjected to renal ischemia-reperfusion injury 1 mo postrecovery (V) compared with control kidneys (U). |
|
Obstruction-induced luminal stretch increases cilia beat frequency. (A) Pronephric obstruction at 48 hpf induced an increased cilia beat rate that closely followed tubule luminal stretch with a latency of 15–30 min. (A, Inset) After stabilization of lumen diameter at 0, 30, 90, and 210 min, cilia beat rate was directly proportional to lumen diameter. Error bars represent SEM. (B) Representative images of GFP-positive E11-9 transgenic tubule segment showing distension at time points indicated. |
|
foxj1a is required for cilia motility. (A) Control scorpion-eGFP-labeled spinal canal cilia in 24-hpf embryos beat rapidly as seen in high-speed line scanning over time (16.9 beats/s in this example; total time, 768 ms. (Scale bar, 100 ms.) (A, Inset) Single cilia imaged in slow-frame scan appears as squiggled line pattern; red line in Inset indicates line acquired in high-speed line scan. (B) High-speed line scan of foxj1a 24-hpf morphant spinal cord cilia shows no motility (total time, 768 ms). (Scale bar, 100 ms.) (B, Inset) Single cilia imaged in slow frame scan shows no movement; red line in inset indicates line acquired in high-speed line scan. (C) Control Kupffer′s vesicle cilia (12-somite stage) beat rapidly, as seen in high-speed line scanning over time (27 beats/s in this example; total time, 768 ms). (Scale bar, 100 ms.) (C, Inset) Single cilia at edge of Kupffer′s vesicle imaged in slow frame scan appears as squiggled line pattern; red line in Inset indicates line acquired in high-speed line scan. (D) High-speed line scan of foxj1a morphant Kupffer′s cilia (12-somite stage) shows no motility (total time, 768 ms). (Scale bar, 100 ms.) (D, Inset) Single cilia imaged in slow frame scan shows no movement; red line in inset indicates line acquired in high-speed line scan. PHENOTYPE:
|
|
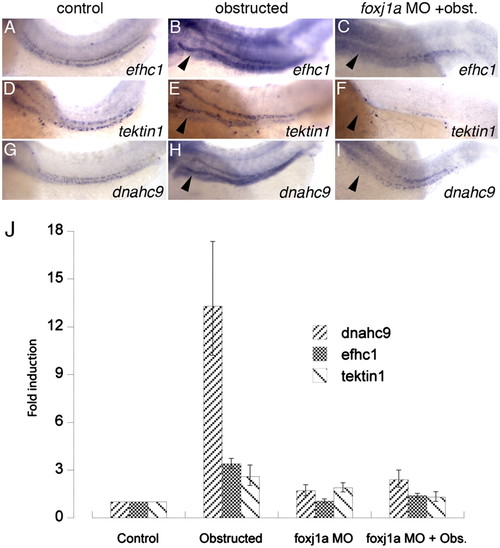
foxj1a target genes are up-regulated in response to tubule obstruction. Control expression of foxj1a target genes efhc1 (A), tektin-1 (D), and dnahc9 (G) at 56 hpf is up-regulated by distal pronephric obstruction (B, E, and H). foxj1a knockdown prevented up-regulation in response to tubule obstruction (C, F, and I). Quantitative real-time RT-PCR (J) confirmed up-regulation of foxj1a target gene expression in obstructed tubules (dnahc9 13.3-fold; efch1 3.4-fold, and tektin1 2.6-fold) and inhibition of induction in foxj1a-deficient obstructed tubules. Error bars in J represent range of minimum and maximum fold foxj1a induction relative to control embryos (comparative threshold cycle method) (59). |
|
foxj1a and foxj1b expression in ciliated cell types at different developmental stages. (A and B) foxj1a is uniformly expressed in precursors to the kidney and spinal cord at the 12-somite stage, as well as in Kupffer’s vesicle (arrowhead). (C) At 24, hpf foxj1a is expressed throughout the spinal cord (arrow) and in all cells of the paired pronephric ducts (arrowhead). (D) By 48 hpf, foxj1a is down-regulated in singly ciliated cells of the pronephros but is maintained in a “salt-and-pepper” pattern in tubule cells, consistent with expression in multiciliated cells. (E) At 48 hpf, expression is also observed in the olfactory placode (arrowhead) and brain ventricular system (arrow). (F) Spinal cord is positive for foxj1a at 48 hpf. (G and H) foxj1b is expressed in developing otic placodes (arrowheads) at the 12-somite stage, and (I) in pronephric multiciliated cells (arrowhead) at 48 hpf. Note that foxj1b expression is seen only at the most distal tip of the spinal cord (arrow). EXPRESSION / LABELING:
|
|
In spinal cord injury, foxj1a but not foxj1b is up-regulated. (A) foxj1a is expressed in the developing spinal cord, and is up-regulated 8 h after mechanical cord injury (arrowhead) induced with a forceps (B). Histological sections of control spinal cord (C) and injured spinal cord (D) reveals an injury-mediated increase in foxj1a in ciliated spinal cord cells. In contrast, foxj1b gene expression remains restricted to multiciliated cells of the pronephric duct in both control (E) and obstructed (F) embryos, and likewise remains restricted to the distal tip of the spinal cord in both spinal cord-intact (G) and spinal cord-injured (H) embryos. |
|
Induction of foxj1a expression by obstruction does not require translation of foxj1a mRNA. (A) foxj1a expression in control 48 hpf embryos is concentrated in multiciliated cells and not in proximal tubule single ciliated cells. (B) foxj1a mRNA expression was up-regulated in foxj1a knockdown embryos (combination of ATG and splice donor-blocking morpholino oligos). (C) Knockdown of foxj1a translation did not prevent up-regulation of foxj1a mRNA by obstruction, arguing against an autoregulatory induction mechanism. |
|
foxj1a and rfx2 are up-regulated in zebrafish cystic kidney mutants. (A and B) In WT embryos at 56 hpf, both foxj1a and rfx2 were expressed in a “salt-and- pepper” distribution characteristic of multiciliated pronephric duct cells. Expression of foxj1a and rfx2 was up-regulated in all cells of the pronephric duct in the dilated pronephric ducts (arrowheads) of 56-hpf embryos homozygous for fleer (C and D), oval (E and F), tg292a (G and H), and double bubble (J) mutations, all of which developed pronephric cysts by 56 hfp, as well as in pkd2 morphants (I). No increase in foxj1a expression over control was observed in pronephros of mutants before cystic stretch (36 hpf,) shown here for the schmalhans cilia cystic mutant (K and L). |
|
foxj1a and foxj1b morphants exhibit distinct ciliopathy phenotypes. (A) Genomic structure of the zebrafish foxj1a gene and sites of ATG and splice donor (SD) MO used in this study. SD MO results in complete deletion of exon 3 as determined by sequencing. (B) RT-PCR analysis of control (WT) and foxj1a SD morphant embryo RNA, indicating complete loss of normal foxj1a mRNA induced by mis-splicing in foxj1a SD morphants. (C) Control morpholino-injected zebrafish at 48 hpf compared with (D) zebrafish injected with a mixture of both foxj1a ATG and SD MO, which uniformly exhibited ventrally curved body axis, hydrocephalus, and normal otolith number (two per otic placode), as well as (E) glomerular cysts (arrowhead) and dilated pronephric tubules (arrows). foxj1a morphants showed frequent situs inversus, as determined by assessment of heart-looping (F; error bars represent SD of three averaged experiments; n = 98 embryos total) and bilateral expression of southpaw (G). (H) Intron-exon boundaries for zebrafish foxj1b and the exon 2 SD MO target site. RT-PCR demonstrated complete loss of WT mRNA and deletion of exon 2 in foxj1b morphants (I). (J) foxj1b Morphants appeared relatively normal, with the exception of abnormal otolith number (K; arrowhead depicting otic placode with three otoliths present). (L) foxj1a/b double knockdown embryos demonstrate curved body axis, kidney cysts, hydrocephalus, and abnormal otolith number. (M) Quantification of abnormal otolith number in foxj1a, foxj1b, and foxj1a+b double morphants. Error bars represent standard deviation of three averaged experiments). |
|
efhc1, tektin-1, and dnahc9 expression in the pronephros is foxj1a and foxj1b dependent. In situ hybridization analysis in control, foxj1a morphant (MO), foxj1b MO, and foxj1a+b double morphants at 24 hpf demonstrated that combined foxj1a/b double knockdown uniformly abolished expression of cilia motility associated genes but not other kidney specific markers or foxj1a itself. (A-D) EF-hand containing domain 1 (efhc1), (E-H) tektin-1, (I-K) dynein heavy chain 9 (dnahc9), (M-P) pax2a, (Q-T) shippo, (U-X) foxj1a (n > 15 embryos for each condition). (A, E, I, M, Q, U) Control embryos; (B, F, J, N, R, V) foxj1a MO; (C, G, K, O, S, W) foxj1b MO; (D, H, L, P, T, X) combined foxj1a/b knockdown. |