- Title
-
Zebrafish enhancer detection (ZED) vector: A new tool to facilitate transgenesis and the functional analysis of cis-regulatory regions in zebrafish
- Authors
- Bessa, J., Tena, J.J., de la Calle-Mustienes, E., Fernández-Miñán, A., Naranjo, S., Fernández, A., Montoliu, L., Akalin, A., Lenhard, B., Casares, F., and Gómez-Skarmeta, J.L.
- Source
- Full text @ Dev. Dyn.
|
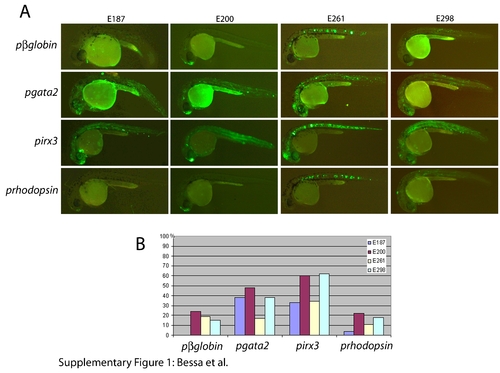
Capacity of irx3 and gata2 promoters to respond to different enhancers. Two or more independent F1 stable transgenic lines were generated with the four tested enhancers (E187, E200, E261, and E298, from left to right columns) positioned 5′ of either the irx3 (upper row) or the gata2 (lower row) minimal promoters, both driving green fluorescent protein (GFP) expression. Both irx3 and gata2 minimal promoters respond efficiently to all four enhancers. However, the irx3 promoter shows some promoter-specific expression in somites and eye (arrows). |
|
The mouse “GAB” and the chicken “5′HS4” insulators are functional in zebrafish. We have generated a new Tol2 transposon-based vector to test the insulator potential of candidate sequences. This vector contains a strong midbrain-specific enhancer (Z48) 5′ of the Cardiac Actin promoter. A: These two components drive GPF expression in the midbrain (Pale orange bracket), due to activity of the Z48 enhancer, and in the somites (pale blue open bracket), due to an endogenous activity of the Cardiac Actin promoter. B: If a strong insulator is placed between the enhancer and the promoter, the midbrain expression should be reduced. C-E: When we placed either the mouse GAB or the chicken 5′HS4 insulators in this vector, we observe that the ratio between midbrain and muscle expression is decreased (D,E) compared with that observed in controls bearing the Gateway entry sequence of 1.7 kb, which has no insulator activity (C). For each insulator, the reduction of the midbrain enhancer activity was observed in 45-65% of the injected embryos (n > 100). |
|
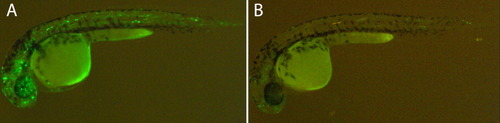
GAB and 5′HS4 insulators decrease the position effect. A: F0 embryos injected with a Tol2 transposon containing the gata2 minimal promoter driving green fluorescent protein (GFP). The GFP background expression is due to position effect in independent random insertions and was observed in a large number of cells in 40% of the injected embryos (n > 100). B: Most of this background is lost in embryos injected with similar construct flanked GAB and 5′HS4 insulators, as we observed GFP expression in only 16% of the injected embryos (n > 100) and in a much reduced number of cells. |
|
The Cardiac Actin-RFP cassette is effective as an internal control of transgenesis. The Cardiac Actin-RFP cassette drives expression of RFP in the somites. A: The broad RFP expression in the muscles of this embryo indicates high efficiency of integration. B: In this embryo, the transgenesis procedure was less effective. Thus, the possibility of germ line integrations is reduced. |
|
A: Diagram of the Zebrafish Enhancer Detection (ZED) vector. Orange boxes are the Tol2 transposase recognition sequences. This vector is composed of two different cassettes. The transgenesis internal control cassette is composed of the Cardiac Actin promoter (pale blue arrow), and red fluorescent protein (RFP; red block). The enhancer detection cassette contains a Gateway entry site, represented by a yellow box, the gata2 minimal promoter, shown in pale blue arrow, and the enhanced green fluorescent protein (EGFP) reporter gene, marked with a Green box. This enhancer detection cassette is flanked by two Insulator sequences represented by Violet circles that protect the enhancer detection cassette (dashed purple box) from position effects. The left insulator corresponds to the GAB insulator (G Ins) from the mouse tyrosinase gene and the right to the 5′HS4 insulator (B Ins) from the chicken β-globin gene. Additionally, two excision cassettes are also present. One is mediated by Flipase (black triangles), and the other by Cre recombinase (gray triangles). B: F0 injected embryos with the ZED vector containing the Z48 enhancer at 48 hours postfertilization (hpf) show both GFP expression in the midbrain (Green) and RFP expression in the somites (Red) in 70% of the injected embryos (n > 150). |
|
The Zebrafish Enhancer Detection (ZED) vector effectively detects enhancer activity. For each construct, the number of injected embryos was higher than 100. A: Green fluorescent protein (GFP) expression in 24 hours postfertilization (hpf) mosaic F0 embryos injected with the ZED vector harboring the E187, E200, E261, E298, Z176 and Z48 enhancers and F1 embryos derived from the Z176 and Z48 constructs. Insets show GFP and red fluorescent protein (RFP) expression in these two last F1 stable lines at 48 hpf. Note the reduced background in these injected embryos as compared to those injected with the noninsulated pgata2 constructs shown in Supp. Fig. S1. For the F1 lines, we obtained more than two independent insertions that showed the same pattern. B: Graphic representation of the percentage of embryos showing GFP expression in the expected territories. The reduced background observed with the ZED constructs did not compromise the enhancer detection potential of this vector as the GFP activity was detected in 30-50% of the injected embryos (n > 100). These percentages are similar to that observed in embryos injected with the noninsulated pgata2 constructs containing the same enhancers (Supp. Fig. S1). |
|
In vivo excision of the internal control and enhancer detection cassettes of the Zebrafish Enhancer Detection (ZED) vector using Flipase and Cre recombinases. A: ZED F1 stable transgenic embryos showing strong red fluorescent protein (RFP) expression in the muscles were injected with Flipase mRNA. This injection caused reduction of RFP expression in some cells of the right side of the embryo (arrow) but not in the left side. RFP reduction upon Flipase mRNA injection was observed in 83% of the injected embryos (n > 200). B: Embryos from a F1 Z48-ZED stable transgenic line. C: Injection of Cre mRNA in these stable transgenic embryos from this line caused mosaic loss of GFP expression. The transition from the homogeneous expression promoted by stable insertion to a mosaic pattern due to Cre-mediated recombination was observed in 85% of the injected embryos harboring the transgene (n > 200). |
|
|