- Title
-
The proneural gene ascl1a is required for endocrine differentiation and cell survival in the zebrafish adenohypophysis
- Authors
- Pogoda, H.M., von der Hardt, S., Herzog, W., Kramer, C., Schwarz, H., and Hammerschmidt, M.
- Source
- Full text @ Development
|
pia encodes the achaete-scute homologue Ascl1a. (A) (Top) Genetic map of a region of linkage group 4 (LG4), showing positions of the piat25215 mutation, the ascl1a gene and some markers used for mapping. Genetic distances of markers from the top of LG4 according to (http://zfin.org/cgi-bin/mapper_select.cgi) are indicated. (Bottom) Schematic representation of Ascl1a protein: the basic helix-loop-helix domain (blue and green) and the piat25215 mutation (red) are indicated. (B) Anti-Myc western blot, showing that translation of chimeric ascl1a-myc mRNA is efficiently blocked in the presence of ascl1a MO (upper panel). The same blot was probed with an anti-pan-cadherin antibody for loading control (lower panel). (C-H) Whole-mount in situ hybridization detecting expression of prl (26 hpf; frontal view, dorsal up; C-E) and pomc (48 hpf; dorsal view, anterior towards the left; F-H) in wild-type (wt; C,F), piat25215 mutant (pia; D,G) and ascl1a morphant embryos (ascl1aMO; E,H). (F-H) pomc-positive cells of the adenohypophysis are indicated with arrowheads, pomc-positive cells of the arcuate nucleus in the hypothalamus are indicated with arrows. (I-K) In situ hybridization at 48 hpf for the pituitary marker prl (p) and the epiphysis marker opsin (o). Injected BAC DNA usually does not distribute uniformly, but leads to chimeric embryos with only a subset of cells containing the injected DNA. Accordingly, the rescued pia embryo in K displays strong prl expression (arrow), but still lacks opsin expression in epiphysis, another phenotypic trait caused by loss of Ascl1a function (Cau and Wilson, 2003). The genotype of the embryo was further confirmed via PCR (see Materials and methods). (L-N) elavl3 in situ hybridization at 11 hpf, dorsal views, anterior towards the left, demonstrating that wild-type Ascl1a fused to the VP16 transactivation domain can induce primary neurons in inter-proneural domains of the neural plate (M), whereas a fusion between VP16 and the truncated Ascl1a protein encoded by the piat25215 allele is ineffective (N). hpf, hours post fertilization. EXPRESSION / LABELING:
|
|
ascl1a is expressed in the adenohypophysis and the adjacent diencephalon. All panels show double (A-F) or single (G-L) in situ hybridization with probes indicated in the lower right-hand corners and ages of specimen in the upper right-hand corners. (B,D,F) Same embryos as in (A,C,E), after the red staining has been washed out. (A-F) Dorsal views, anterior towards the left; (G-L) lateral views, anterior towards the left, dorsal upwards. Arrows indicate adenohypophysis (G-L) or its anlage at the anterior neural ridge (A-F). ah, adenohypophysis; nh, neurohypophysis; pvh, posterior-ventral hypothalamus. EXPRESSION / LABELING:
|
|
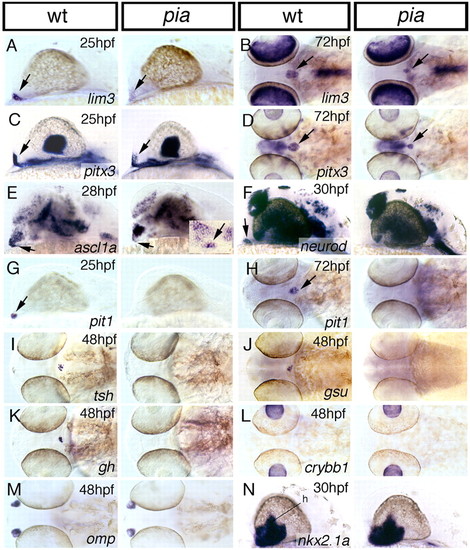
ascl1a mutants show decreased expression of the lim3, pitx3 and ascl1a, and fail to initiate expression of neurod, pit1 and all adenohypophyseal hormone genes, whereas the hypothalamus appears unaffected. Columns 1 and 3 show wild-type siblings (wt); columns 2 and 4 show pia mutants. Probes used for whole-mount in situ hybridization are indicated in lower right-hand corners, ages of embryos in the upper right-hand corners of wild types. (A,C,E,F,G,N) Lateral views, anterior towards the right, dorsal upwards. (B,D,H-M) Dorsal views, anterior towards the right. Arrows in A-H indicate expression in the adenohypophysis. Inset in E shows section through pituitary of pia mutant at 120 hpf (see Fig. 2J for wild-type control). h, hypothalamus. EXPRESSION / LABELING:
|
|
pia mutants display indistinct early pituitary morphology, followed by a transient phase of adenohypophyseal cell death and the formation of a smaller, but distinct, pituitary gland with cells of rather primitive ultrastructure. (A-L) Nomarski images of live pia mutant embryos (pia) and their corresponding wild-type siblings (wt). (F,H,I-L) Images are superimposed by Acridine Orange staining for apoptosis. (A,B,E-J) Frontal views, dorsal upwards; (C,D) lateral views, anterior towards the left, dorsal upwards; (K,L) ventral views, anterior towards the right. Ages of embryos are indicated in upper right-hand corners of wild types. Genotypes were determined via PCR after photography. Arrowheads in A-J indicate borders of the pituitary gland; in K,L, pituitary borders are outlined by dots. (M-T) Pituitary ultrastructure at 120 hpf; longitudinal sections, anterior towards the left, dorsal towards the top. (M,O) Toluidine Blue staining; (N,P-T) electron micrographs. (M-P) The border of the pituitary is indicated by arrows; (N,P) the border between adenohypophysis (ah) and neurohypohysis (nh) is outlined by black dots. (Q-T) Higher magnifications of regions indicated by red arrows in N,P. Vesicles (as in T, indicated by arrows) were seen in three out of ~20 adenohypophyseal cells present in the section of the pia pituitary (P). They could contain matrix proteins and hormone-binding proteins, which can be made even in the absence of hormone production (compare with Norris, 1997). Scale bars: 30 μm in M-P; 1 μm in Q-T. |
|
Ascl1 is required cell autonomously to promote pit1 expression, cell survival and Prl production. (A-C) Lateral views on chimeric embryos at 26 hpf embryos, in situ hybridized for pit1 transcripts in blue and with transplanted wild-type cells in brown (indicated with arrows). (A) Wild-type control recipient with wild-type donor cells in the telencephalon and normal pit1 expression. (B) pia recipient with wild-type cells in eyes, telencephalon and ventral regions of diencephalon, lacking adenohypophyseal pit1 expression. (C) pia embryo with few wild-type cells within adenohypophyseal domain, showing pit1 expression in these wild-type cells only. (D-F) Frontal views of pituitaries at 32 hpf. Nomarski images superimposed with red fluorescence revealing transplanted cells; green fluorescence revealing Acridine Orange (AO)-positive cells. (D) Wild-type control recipient with AO-negative wild-type donor cells in the pituitary. (E) pia recipient, with AO-negative transplanted wild-type cells (red), but many AO-positive host cells (green) in pituitary. (F) Wild-type recipient, with one AO-positive (yellow) and two AO-negative ascl1a morphant donor cells in pituitary. (G-I) Ventral views of pituitaries at 48 hpf, anterior towards the left. Left panels show transplanted h2a::h2a-GFP transgenic cells weakly labeled in green, right panels an overlay of the same images with anti-Prl immunostaining in red. (G) Wild-type host; (H) pia host without transplanted cells in pituitary; (I) pia host with three wild-type cells in pituitary, two of which produce Prl protein. |
|
Fgf3 is required for adenohypophyseal ascl1a expression and requires Ascl1a to induce pit1 expression. (A-D) Whole-mount in situ hybridization (lateral views with anterior towards the right) showing that adenohypophyseal ascl1 expression (indicated by arrows in A,B) is lacking in fgf3/lia mutants at 28 hpf, while diencephalic fgf3 expression (indicated by arrows in C,D) is normal in ascl1a/pia mutants at 32 hpf. (E-J) Frontal views on embryos at 28 hpf, after implantation of Fgf3-soaked beads in embryos of indicated genotype, and in situ hybridization for ascl1a (E-G) or pit1 (H-J). Beads in E,F,H-J are marked with arrowheads. Arrows in E,F mark laterally expanded ascl1a expression; asterisks in E-G indicate medial adenohypophyseal ascl1a expression, which is much stronger in the pia mutant after Fgf3 bead implantation (F) compared with untreated pia sibling (G). |

Unillustrated author statements EXPRESSION / LABELING:
|