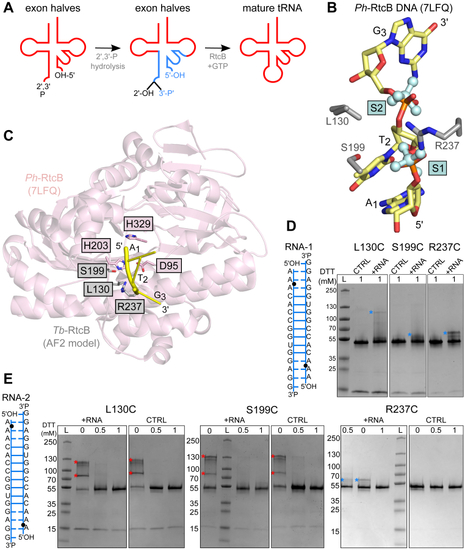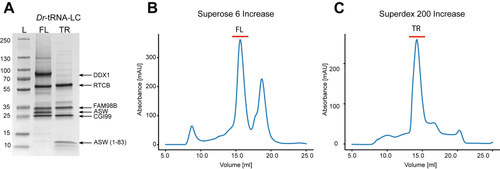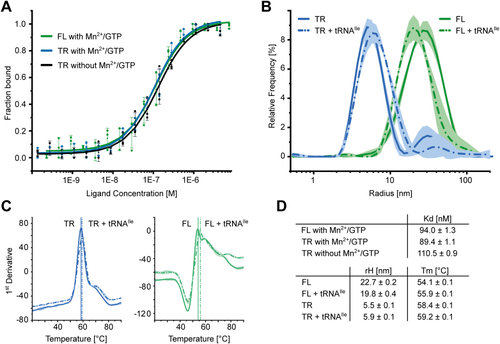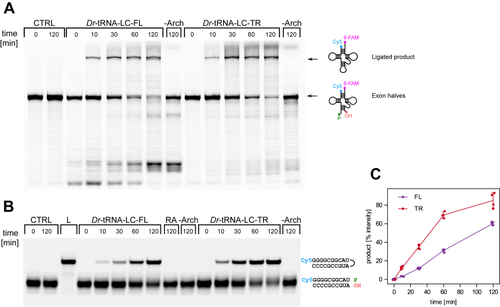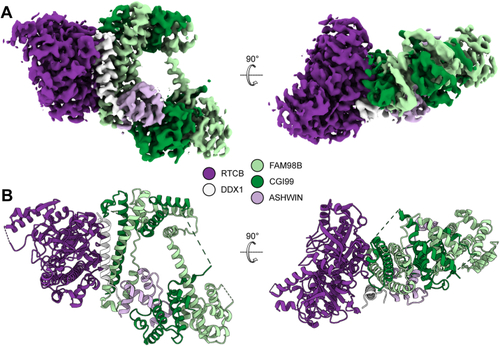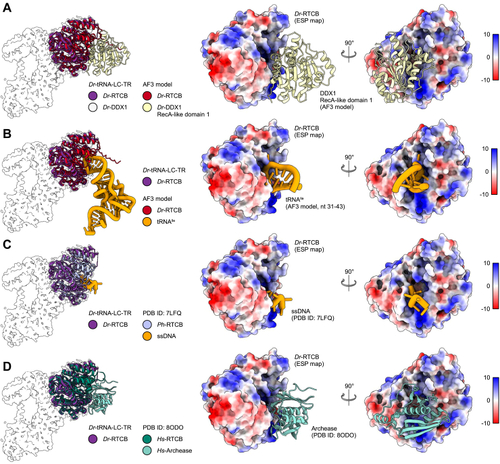
The position of DDX1 subunit in Dr-tRNA-LC.A, superimposition of the AlphaFold 3 model of the Dr-tRNA-LC-FL on Dr-RTCB from the Dr-tRNA-LC-TR structure. Left, Dr-RTCB and Dr-DDX1 shown on the contour of the Dr-tRNA-LC-TR map. Right, modeled interaction between DDX1 RecA-like domain 1 (AlphaFold 3 model) and Dr-RTCB (shown as Coulombic electrostatic potential surface); two views. B, superimposition of the AlphaFold 3 model of the Dr-tRNA-LC-TR-tRNAIle on Dr-RTCB from the Dr-tRNA-LC-TR structure. Left, Dr-RTCB and tRNAIle shown on the contour of the Dr-tRNA-LC-TR map. Right, modeled interaction between tRNAIle (AlphaFold 3 model; only anticodon loop is shown for clarity) and Dr-RTCB (shown as Coulombic electrostatic potential surface); two views. C, superimposition of Ph-RtcB in a complex with ssDNA (PDB ID: 7LFQ) on Dr-RTCB from the Dr-tRNA-LC-TR structure. Left, Dr-RTCB, Ph-RTCB, and ssDNA shown on the contour of the Dr-tRNA-LC-TR map. Right, modeled interaction between ssDNA and Dr-RTCB (shown as Coulombic electrostatic potential surface); two views. D, superimposition of Hs-RTCB in complex with Archease (PDB ID: 8ODO) on Dr-RTCB from the Dr-tRNA-LC-TR structure. Left, Dr-RTCB, Hs-RTCB, and Hs-Archease shown on the contour of the Dr-tRNA-LC-TR map. Right, modeled interaction between Archease and Dr-RTCB (shown as Coulombic electrostatic potential surface); two views. Dr-tRNA-LC, ligase complex from Danio rerio; FL, full length; Hs, Homo sapiens; PDB, Protein Data Bank; Ph-RtcB, Pyrococcus horikoshii; TR, truncated version.
|