- Title
-
Oxygen chemoreceptor inhibition by dopamine D2 receptors in isolated zebrafish gills
- Authors
- Reed, M., Jonz, M.G.
- Source
- Full text @ J. Physiol.
|
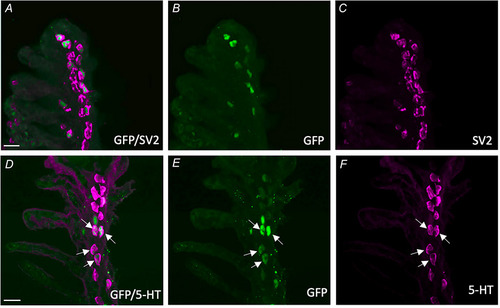
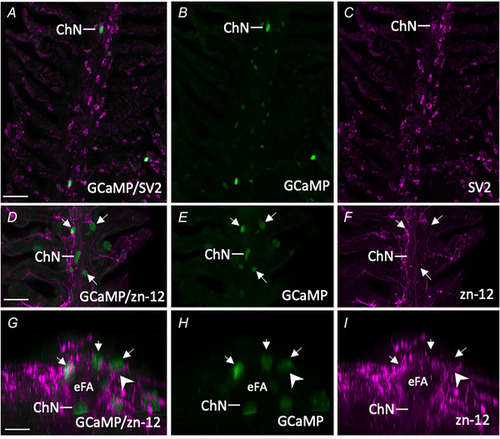
Characterization of GCaMP‐positive neuroepithelial cells (NECs) in the gill epithelium of transgenic Confocal imaging of immunohistochemical localization of GCaMP with NECs containing synaptic vesicle protein‐2 (SV2) and 5‐hydroxytryptamine (5‐HT). |
|
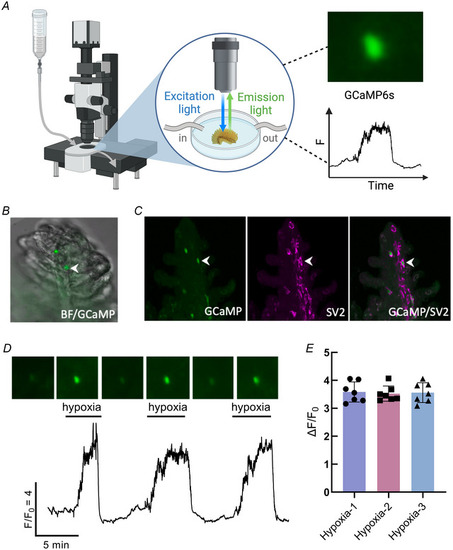
Hypoxia induced intracellular Ca2+ responses in gill neuroepithelial cells from Tg( |
|
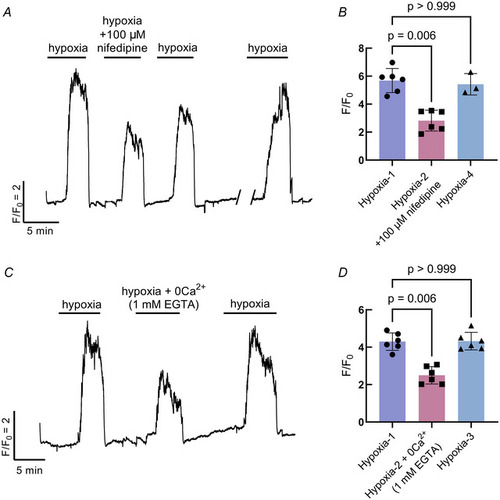
Extracellular Ca2+ contributes to the response to hypoxia in neuroepithelial cells (NECs) |
|
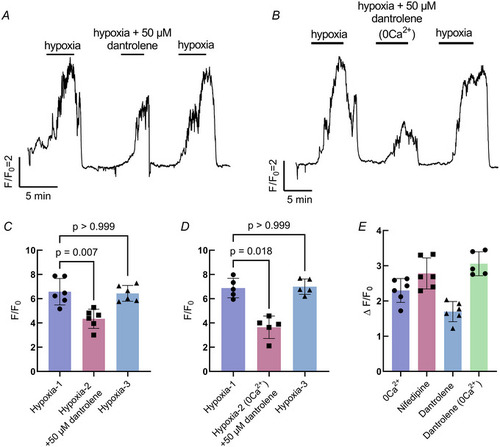
Intracellular Ca2+ contributes to the response to hypoxia in neuroepithelial cells (NECs) |
|
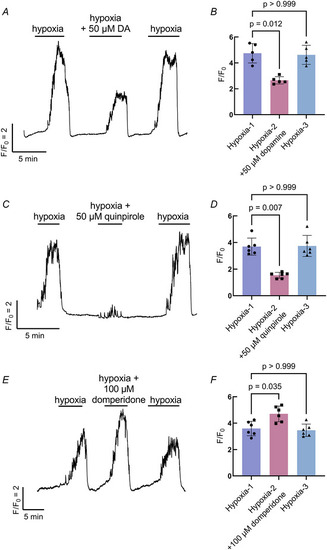
The effects of D2R activity on the neuroepithelial cell (NEC) response to hypoxia Showing the effects of dopamine ( |
|
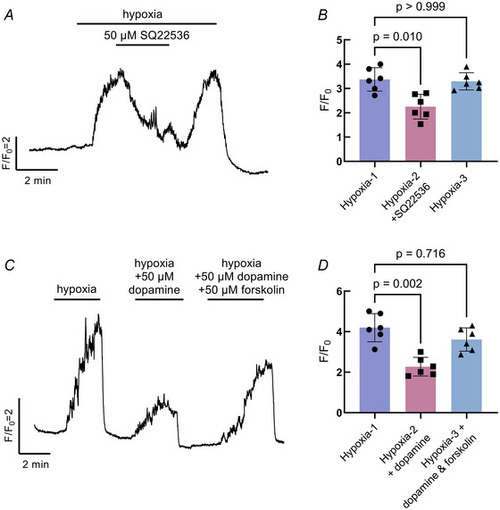
Dopamine acts through intracellular secondary messenger cAMP in neuroepithelial cells (NECs) |
|
Characterization of GCaMP‐positive postsynaptic chain neurons (ChNs) in Tg( |
|
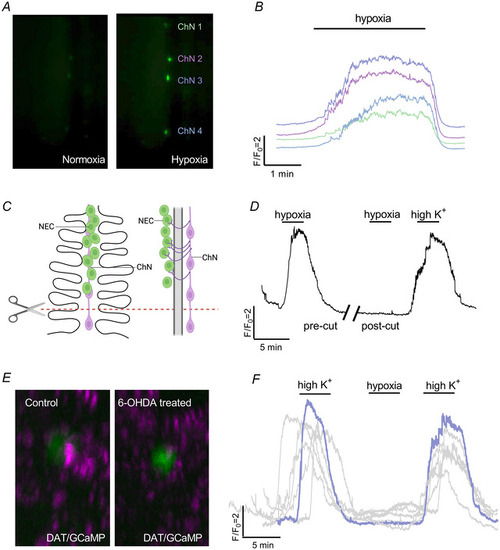
The chain neuron (ChN) calcium response to hypoxia requires synaptic contact with neuroepithelial cells (NECs) |
|
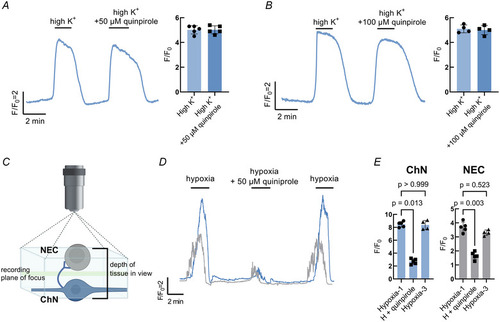
Postsynaptic modulation of the hypoxic response by presynaptic D2R activation |