- Title
-
Disruption of zebrafish somite development by pharmacologic inhibition of hsp90
- Authors
- Lele, Z., Hartson, S.D., Martin, C.C., Whitesell, L., Matts, R.L., and Krone, P.H.
- Source
- Full text @ Dev. Biol.
|
Specific interaction of zebrafish Hsp90 with solid-phase geldanamycin (GA). Using Western blot analysis with anti-Hsp90 antibody, Hsp90 was detected to bind to GA-derivatized resin (B, lane 1) and this interaction was completely inhibited by preincubation with free GA prior to application to the GA resin (lane 2). Zebrafish Hsp90 comigrated with rabbit reticulocyte lysate Hsp90 detected using the same antibody (lane 3). No other proteins interacted with the resin in a specific manner as determined by silver staining of SDS–polyacrylamide gels (compare lanes 1 and 2 of A). |
|
In vitro-translated Hsp90α and Hsp90β interact with GA-derivatized resin in a specific manner. Coupled in vitro transcription/translations were carried out and the 35S-labeled products subjected to GA-affinity column analysis as described under Materials and Methods. Both translation products, which have an Mr consistent with their identity as Hsp90 (A), bind to GA-derivatized resin (B) in a manner which is competed with free GA but not DMSO (compare lane 2 to lane 1 and lane 4 to lane 3). |
|
Expression of hsp90α during early somite development as detected by in situ hybridization analysis. (A) 3- to 4-somite stage, dorsal view. (B) 16 somite stage, dorsal view. (C) Cross section through unsegmented paraxial mesoderm prior to somite formation. (D) Longitudinal section through the dorsal–ventral midline at the location of the most recently formed somite. Strong constitutive expression of hsp90α is confined to the adaxial cells (closed arrows). Very low levels of hsp90α mRNA are sometimes detectable in the lateral presomitic cells which lie distal to the adaxial cells (C). Open arrows in D indicate the most recently formed somite furrow. n, notochord. Scale bar in A and B, 100 μm. Scale bar in C and D, 50 μm. |
|
Expression of hsp90β in zebrafish embryos as detected by in situ hybridization analysis. (A) Sphere stage. (B) 30% epiboly. (C and D) Bud stage. (E–H) 20 h. (A, B, D, and E) Lateral view. (C) Ventral view. (F) Dorsal view. (G and H) Cross section through the forebrain and midbrain regions, respectively. hsp90β expression is widespread in blastula-stage embryos (A and B). During late gastrulation and early somitogenesis, hsp90β mRNA is detectable predominantly in the anterior structures of the embryo (C and D). At 20 h of development, the strongest expression of hsp90β is within the CNS, particularly in cells along the central canal and the exterior margins (open arrows in F and H) as well as the developing retina (G). In addition, moderate expression is observed in the tail bud and in cells immediately caudal to the yolk ball extension (triangle in E). No hsp90β mRNA is detectable in the notochord (asterisk in E). Scale bar, 100 μm. |
|
Trunk/tail phenotype of 18-h-old embryos treated with geldanamycin beginning at 50% epiboly (5–5.5 h). (A, C, and E) Control. (B, D, and F) GA. (A–D) Lateral view of whole embryos. (E and F) Longitudinally oblique sections through the four most recently formed somites and the unsegmented paraxial mesoderm. (A, B, E, and F) Unstained. (C and D) In situ hybridization detection of α-tropomyosin mRNA. Anterior is to the left in all. Seventy-eight percent of the GA-treated embryos exhibited a shortened trunk/tail phenotype and the somites did not form their characteristic full chevron shape as described under Results (compare arrows in C and D, which indicate somites at comparable positions along the anterior–posterior axis). In addition, somites of treated embryos typically assumed a block-like shape and varied significantly in size within any one embryo (compare somites indicated by asterisks in E and F, which are the four most recently fully formed in both cases). The closely related benzoquinone ansamycin geldampicin, which is ineffective as an Hsp90-binding agent, had no detectable effect on embryonic development. In contrast to embryos treated with GA beginning at gastrulation, treatment initiated at the blastula stage was rapidly lethal. Scale bar in A–D, 100 μm. Scale bar in E and F, 25 μm. |
|
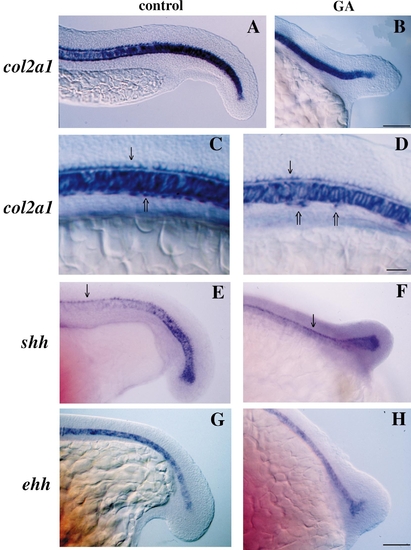
Expression of notochord and floor plate markers is normal in GA-treated embryos as detected using whole-mount in situ hybridization. (A, C, E, and G) Control. (B, D, F, and H) GA treatment beginning at 50% epiboly. (A–D) col2a1 mRNA. (E and F) shh. (G and H) ehh. Anterior is to the left and dorsal to the top in all. All three markers were expressed normally in the notochord of GA-treated embryos as were other markers of notochord formation such as axial and ntl (data not shown). As well, a single, uninterrupted row of floor-plate cells which express both col2a1 and shh developed immediately above the notochord in both control and GA-treated embryos (arrows in C–F). However, treated embryos exhibited large gaps in the row of col2a1-expressing hypochord cells (open arrows in D) which normally lie as a continuous row immediately below the notochord (open arrow in C). Control embryos in A and C are at the 23- to 24-somite stage and 15-somite stage in E and G. GA-treated embryos are the same age as control embryos. Scale bar in A, B, and D–G, 100 μm. Scale bar in B and C, 50 μm. |
|
GA and forskolin inhibit formation of eng-2-expressing muscle pioneer cells. (A and D) Control. (B and E) GA treatment beginning at 50% epiboly. (C and F) Forskolin treatment beginning at 50% epiboly. (A–C) Whole 18-h-old embryos. (D–F) Two anterior somites at comparable positions within the trunk of these embryos. In situ hybridization for the detection of eng-2 mRNA was carried out as described under Materials and Methods. All embryos are lateral views with anterior to the left and dorsal to the top. Muscle pioneers, which can be distinguished from other striated muscle cells by high levels of eng-2 expression, normally develop at the D–V midline of the myotome at the future location of the horizontal myoseptum (arrows in A and D) but are absent from this location in GA-treated and forskolin-treated embryos (arrows in E and F). However, activation of eng-2 expression in cells of the midbrain/hindbrain boundary is unaffected by GA or forskolin treatment (asterisks in A–C). Scale bar in A–C, 100 μm. Scale bar in D–F, 20 μm. |
|
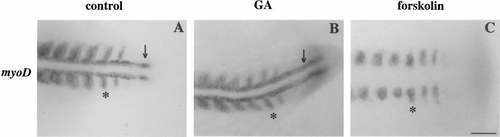
Adaxial cells form in GA-treated but not in 14-h-old forskolin-treated embryos. (A) Control. (B) GA. (C) Forskolin. Adaxial cells are demonstrated based on their early, strong expression of myoD. Adaxial cells (arrow) could be detected directly adjacent to the notochord prior to somitogenesis in both control and GA-treated embryos but did not form in embryos treated with forskolin. In contrast, the myoD-expressing lateral presomitic cells which will go on to form fast muscle fibers were normal in all three groups of embryos (asterisks in A–C). Treatment with GA and forskolin was initiated at 50% epiboly. Scale bar, 50 μm. |
Reprinted from Developmental Biology, 210(1), Lele, Z., Hartson, S.D., Martin, C.C., Whitesell, L., Matts, R.L., and Krone, P.H., Disruption of zebrafish somite development by pharmacologic inhibition of hsp90, 56-70, Copyright (1999) with permission from Elsevier. Full text @ Dev. Biol.