- Title
-
Characterisation of a second patched gene in the zebrafish Danio rerio and the differential response of patched genes to hedgehog signalling
- Authors
- Lewis, K.E., Concordet, J.P., and Ingham, P.W.
- Source
- Full text @ Dev. Biol.
|
Wild-type expression of ptc and hh genes during early development. Comparison of ptc and hh gene expression during the first 18 h of wild-type zebrafish embryogenesis. Transcripts were revealed by in situ hybridisation with antisense RNA probes to ptc1 (B, F, I, and K); ptc2 (A, E, G, H, and J); shh (C and L), and pax-b + ptc2 (D). (A–C) Dorsal views of embryos at the end of gastrulation (tail-bud stage) with their anteroposterior axis oriented vertically. ptc1 and ptc2 have different expression patterns at this stage; ptc2 is exclusively expressed in the neurectoderm above the midline (A), whereas ptc1 is also expressed in the adaxial cells and the presomitic mesoderm (B). (D–I) Dorsal flat preparations (with the yolk removed). Anterior is to the left. (D) ptc2 and pax-b expression at about 8 somites. pax-b is expressed in the midbrain–hindbrain boundary (shown with an asterisk) and the presumptive otic vesicle which is centred on presumptive rhombomere 5 but extends into presumptive rhombomeres 4 and 6 (indicated with a black square bracket). A stripe of ptc2 expression is visible in presumptive rhombomere 3 and a much weaker stripe of expression is present in rhombomere 5 (indicated with yellow double arrowhead lines); (E and F) ptc2 and ptc1 expression, respectively, at 5–6 somites and (L) a close-up of ptc2 expression in the caudal trunk at this stage. ptc2 is expressed throughout the medial lateral extent of the somites at this stage but ptc1 expression is still confined to the adaxial cells; ptc2 expression in the somites remains broader than ptc1 expression at 10–12 somites and this is shown in close-up (H and I). (J–L) Lateral views of embryos at 18 somites. ptc2 expression (J) is now clearly broader than ptc1 expression (K) in the brain, neural tube, and somites. |
|
Wild-type expression at 24 h postfertilisation. Transcripts were revealed by in situ hybridisation with antisense RNA probes to ptc1 (A, E, H, and I), ptc2 (B, C, D, G, and J), and shh (F) and slow muscle fiber types were detected by immunolocalisation with the BA-D5 antibody (K). All embryos were fixed at 24 hpf. (A and B) Expression in the hindbrain. There are six vertical stripes of ptc2 expression which mark the boundaries of rhombomeres 2–6 ((B) shown with black arrows). This rhombomeric expression of ptc2 is similar, but not identical, to the expression of ptc1 (A), which is elevated in rhombomeres 2 and 4 (rectangular brackets) and the edges of rhombomere 6 (black arrows). The expression of ptc1 and ptc2 differs considerably in the somites; ptc1 is expressed only in the presomitic and somitic mesodermal cells that are adjacent to the notochord, whereas ptc2 has a dynamic pattern of expression along the rostrocaudal axis which is very similar to that of BA-D5 which marks slow muscle fiber types (G–J and cf. K). (C–F) were obtained by embedding embryos in wax and sectioning them after the in situ hybridisation procedure. The black arrows point to the floor plate, the red dashed lines demarcate the ventral edge of the neural tube, and nc denotes notochord. Neither ptc1 nor ptc2 is expressed in the rostral floor plate (C–E), though both are still expressed in the floor plate in the tail (data not shown). The neural tube expression of ptc2 is slightly stronger than the somite expression as revealed by the specimen in (C), which was stained for a shorter time than that in (D): (C) shows ptc2 expression throughout the neural tube except for the floor plate while (D) shows ptc2 expression in the neural tube and throughout the somites. ptc1 expression is much more restricted than ptc2; it is expressed only in the ventral neural tube (apart from the floor plate) and the medial somite (E). Neither ptc gene is expressed in the notochord. (F) Caudal section where shh is still expressed in the notochord, in addition to being expressed in the floor plate. |
|
ptc expression in cyclops homozygotes. Transcripts were revealed by in situ hybridisation with antisense RNA probes to shh (A and E), twhh (B and F), ptc2 (C and G), and ptc1 (D and H). (E–H) show expression in cyclops homozygotes and (A–D) show expression in wild-type siblings processed in parallel. All embryos were fixed at 24 hpf. Expression of both ptc genes is lost from all the areas of the anterior brain except for cells around the cells still expressing shh and/or twhh. |
|
ptc expression in flh homozygotes. Transcripts were revealed by in situ hybridisation with antisense RNA probes to shh (A), ptc2 (B), and ptc1 (C). All panels show lateral views of the trunk of flh homozygotes above the yolk extension, at 24 hpf with anterior to the left. Expression of both ptc genes is lost from the somites and from most of the caudal trunk apart from around the groups of floor plate-like cells that still express shh and twhh. In addition, ptc2 expression extends more dorsally in the neural tube from these groups of cells than ptc1 expression does. |
|
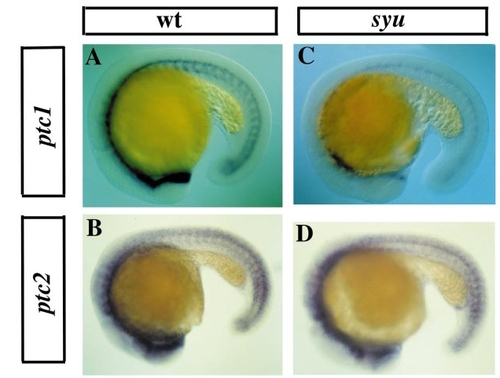
ptc expression in syu homozygotes. Transcripts were revealed by in situ hybridisation with antisense RNA probes to ptc1 (A and C) or ptc2 (B and D) on 18–20 somite embryos. Mutations homozygous for syu have a dramatic reduction in ptc1 expression (C and cf. A, which shows wild-type ptc1 expression), but hardly any reduction in ptc2 expression (D and cf. B, which shows wild-type ptc2 expression). |
|
ptc expression in con, syu, and yot. (A–F) Fin bud expression of ptc1 (A–C) and ptc2 (D–F) at 32 hpf. In all cases the fin buds are indicated with black arrows. Both ptc1 and ptc2 are expressed in wild-type fin buds but the ptc2 domain is both broader and weaker than that of ptc1 (A and D). In contrast neither gene is expressed in the fin buds of syu homozygotes (B and E) and expression of both ptc1 and ptc2 is reduced in the fin buds of con homozygotes (C and F) though ptc1 is affected more than ptc2. (G–J) Lateral views of ptc2 expression with anterior to the left. The rectangular brackets in G and I indicate the equivalent regions of embryos shown in close-up in H and J. At 24 hpf ptc2 expression is only slightly affected, if at all, by any of the “U” class of mutations. In yot homozygotes ptc2 expression is very slightly weaker than in wild-type embryos (I) but less so than in con or syu homozygotes; the one exception to this is in the anterior somites, where expression of ptc2 is eliminated (J). |
|
Induction of ectopic ptc expression by overexpression of shh or inhibition of PKA. Transcripts were revealed by in situ hybridisation with antisense RNA probes to ptc1 (A–G) or ptc2 (H–N). (B, C, E, F, I, J, L, and M) were injected with shh RNA, and (G and N) were injected with dnPKA at the 1–4 cell stage and then fixed at 24 hpf. (A, D, H, and K) show control uninjected embryos that were processed in parallel. Injections of synthetic RNA from all the different hh genes and of a dnPKA construct all gave the same results. High concentrations of injected RNA resulted in ectopic expression of both ptc genes in the heads of injected embryos at 24 hpf (B, C, E–G, I, J, L–N); ptc1 was also often ectopically expressed in the trunks (B shows an example of strong ectopic expression and C shows an example of weaker ectopic expression), but in contrast ptc2 expression was lost from the ventral trunks of the more severely affected embryos (I). |
Reprinted from Developmental Biology, 208, Lewis, K.E., Concordet, J.P., and Ingham, P.W., Characterisation of a second patched gene in the zebrafish Danio rerio and the differential response of patched genes to hedgehog signalling, 14-29, Copyright (1999) with permission from Elsevier. Full text @ Dev. Biol.