- Title
-
Specification of the hindbrain fate in the zebrafish
- Authors
- Woo, K. and Fraser, S.E.
- Source
- Full text @ Dev. Biol.
|
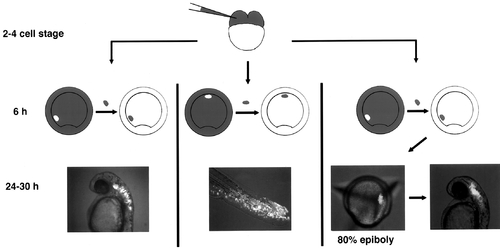
Fate mapping by homotopic transplantation. Fate mapping by single-cell injection indicated that hindbrain progenitors are located at a sector 40–90° from the midline of a shield-stage gastrula. (First panel) Labeled grafts taken from this region and placed into the same position in an unlabeled host gave rise to cells that spanned the entire hindbrain region, as predicted by the fate map. (Second panel) A labeled graft made to the ventral most sector of the ectoderm gave rise to cells in the posterior trunk and tail, which include ectodermal cells, endoderm, and somitic cells. (Third panel) To map the position of hindbrain progenitors at 80% epiboly, labeled cells were orthotopically replaced into the shield-stage embryos, and the location of labeled cells was tracked at 80% epiboly. The majority of the hindbrain progenitors fate map to the middle third of the epiblast, adjacent to the dorsal axis. |
|
The different behaviors of presumptive hindbrain progenitors transplanted at different times to identical positions in 6 h hosts. (A) Presumptive hindbrain progenitors (white) grafted at 6 h to the ventral epiblast region of a 6-h host integrate seamlessly to give rise to trunk and tail epidermis. (B) Presumptive hindbrain progenitors (white; arrow) grafted at 80% epiboly to a 6-h host form a small condensation of cells that does not integrate into the host. (C, closeup in D) Presumptive epidermal progenitors (white) grafted at 80% epiboly to a 6-h host integrate well into the host and gave rise to epidermis. Scale bar: all 100 μm. |
|
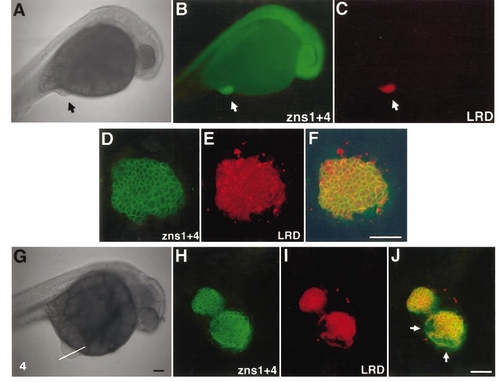
80H cells express neural markers ectopically. (A–C) An embryo with an 80H graft at the lower abdomen region (arrows) viewed in brightfield (A) and after labeling with zns1 and zns4 antibodies (B). These neural-specific antibodies labeled the host’s neural axis (B), as well as the graft, visualized with lineage tracer (LRD; C). (D–F) High-magnification confocal image of the graft seen labeled with rhodamine dextran (E) and zns antibodies (FITC-labeled; D). An overlay of the two (F) shows that all the grafted cells (LRD-positive) are positive for these neural-specific antibodies. (G–J) An example of 80H graft in which host tissue close to the graft also expressed neural antibody, offering evidence of homeogenetic induction. Brightfield image of the host (G) indicating the optical section plane for the confocal images (H–J). In this embryo, the 80H graft (I) had segregated into two parts, interposed with host-derived tissue. Zns1 + 4 antibody revealed cells that were green but not red, indicating that these host-derived cells have adopted neural fate (H, composite image in J). Scale bars: A and G, 100 μm; F and J, 50 μm. |
|
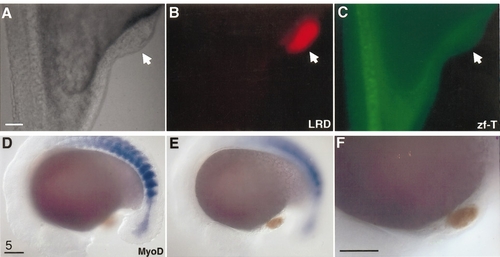
Neither axial nor paraxial mesoderm is present within 80% epiboly hindbrain transplants. (A–C) Zf-T antibody detected host notochord cells (C), but failed to detect any cells within the graft (arrow in all panels). (D–F) In situ hybridization of MyoD (blue) labeled intensely the paraxial mesoderm (presumptive somites) of the host, but failed to give any signal within the graft (orange). All scale bars: 100 μm. |
|
Committed hindbrain cells express coherent patches or stripes of Krox20. (A) A single patch of Krox20 (arrow) is present in the 80HV graft. (B, C) Two distinct stripes of Krox20-positive cells (blue color) are expressed by the 80HV graft. Note that both Krox20 domains (C, arrows) are within the biotin-visualized lineage tracer territory (orange). (D–F) Another example with two distinct domains of Krox20: one stripe of Krox20 is expressed by grafted cells (black arrow); the other patch is expressed by host cells (devoid of lineage label that marks donor cells (green arrow). This host-derived Krox20 domain is continuous with the graft-derived structure extending outward from the yolk. (G, H) Histological sections of different embryos illustrating that graft-derived (black arrows) and host-derived (green arrows) Krox20-expressing cells are intermingled in the same structure. All scale bars: 100 μm. |
|
80H cells can maintain Krox20 expression even in nonnaive environment. (A and B) 80H cells grafted to the animal pole become lodged in the forebrain of the host (A, arrow). The graft is positive for neural markers zns-1 and zns-4 (B, arrow indicates graft). (C and D) In situ hybridization show that 80H graft is able to maintain Krox20 expression, and hence hindbrain identity, in presumptive forebrain territory of a 6-h host. These photos show the same embryo prior to (C1, magnified in C2) and subsequent to (D1, magnified in D2) biotin visualization of the graft, showing that Krox20 expression is entirely contained within the graft. All scale bars: 100 μm. |
|
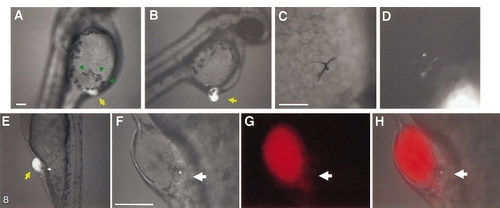
Ectopically located hindbrain progenitors induce ectopic neural crest derivatives and otic vesicles. (A–D) Ectopic pigment cells are detected near 80H grafts of LRD-labeled tissues. (A) Ectopic pigment cells (green arrows) are present near the graft (yellow arrow); normally, the yolk is devoid of large pigment cells at this stage (compare left and right side of the yolk). (B–D) The majority of the ectopic pigment cells are host-derived, but occasionally, rhodamine-labeled (i.e., graft-derived) pigment cells are also seen. (E–H) Ectopic otic vesicles are formed adjacent to 80H grafts. Ectopic otic vesicles (E, magnified views F–H) can form in different regions of the ectoderm overlaying the yolk. (This embryo actually formed a pair of ectopic otic vesicles, one on either side of the graft. All ectopic otic vesicles are host-derived.) |
Reprinted from Developmental Biology, 197, Woo, K. and Fraser, S.E., Specification of the hindbrain fate in the zebrafish, 283-296, Copyright (1998) with permission from Elsevier. Full text @ Dev. Biol.