- Title
-
Csf1rb regulates definitive hematopoiesis in zebrafish
- Authors
- Dai, Y., Wu, S., Cao, C., Xue, R., Luo, X., Wen, Z., Xu, J.
- Source
- Full text @ Development
|
Csf1rb is highly expressed in hemogenic endothelium and HSPCs. (A,B) Diagram at the top shows a 30-36 hpf zebrafish embryo; the orange line indicates the VDA region. (A) WISH of csf1rb in the Tg(flk1:GFP) line at 36 hpf in the VDA region. White arrows indicate colocalization of csf1rb (red) and GFP+ HE (green) with a flat shape. Yellow arrowheads indicate csf1rb-positive naïve HSPCs with a round shape. n=12. (B) WISH of csf1rb in the Tg(cmyb:GFP) line at 30 hpf in the VDA region. Yellow arrowheads indicate colocalization of csf1rb (red) and GFP+ HSPCs (green). White arrowheads indicate cmyb-GFP+ multiciliated cells. n=12. (C,D) Diagram at the top shows a 2 dpf zebrafish embryo; the orange line indicates the VDA region. (C) WISH of csf1rb in the VDA of Tg(cmyb:GFP) at 2 dpf. White arrowheads indicate cmyb-GFP+ multiciliated cells. n=12. (D) WISH of csf1rb in the VDA of Tg(runx1:en-GFP) at 2 dpf. Yellow arrowheads indicate colocalization of csf1rb (red) and GFP+ HSPCs (green). n=15. Brightfield images are two to five layers stacked to show the VDA region (indicated by the red dashed lines). Scale bars: 20 μm. |
|
csf1rb deficiency does not interfere with the formation of HSPCs but leads to HSPC reduction and multiple lineage defects at early developmental stages. (A) Budding behavior of the converted cell in the VDA in siblings (sib) and csf1rb mutants (mut). Orange line in the diagram indicates the VDA region. Upper panels show flk1-NLS-Eos+ green endothelial cells in the VDA; nine cells were converted into red after UV light conversion. Lower panels show the budding behavior of the cell in the white dashed line, enlarged at different time points in the bottom panels and indicated by white arrowheads. The time scale is shown in the left corner. (B) Quantification of the number of cells budding among converted endothelial cells over 26 h in siblings (n=11) and csf1rb mutants (n=8). (C) WISH of cmyb in siblings (n=16) and csf1rb mutants (n=16) at 30 hpf; red arrows show naïve HSPC signal in the VDA. (D) WISH of cmyb in siblings and csf1rb mutants at 2 dpf. (E) Quantification of cmyb+ HSPCs in siblings (n=12) and csf1rb mutants (n=12) in the VDA and the CHT at 2 dpf. (F) WISH of cmyb in csf1rb mutants and siblings at 3 dpf. (G) Quantification of cmyb+ HSPCs in siblings (n=40) and csf1rb mutants (n=16) in the CHT at 3 dpf. (H) WISH of runx1 in siblings and csf1rb mutants at 3 dpf. (I) Quantification of runx1+ HSPCs in siblings (n=20) and csf1rb mutants (n=12) in the CHT at 3 dpf. (J) WISH of αe1-globin in siblings and csf1rb mutants at 5 dpf. (K) Quantification of αe1-globin+ erythrocytes in siblings (n=52) and csf1rb mutants (n=22) in the CHT at 5 dpf. (L) Immunostaining of Lcp1 in the CHT at 5 dpf in siblings and csf1rb mutants. (M) Quantification of the Lcp1+ myeloid cells in siblings (n=11) and csf1rb mutant (n=10) embryos. (N) WISH of rag1 at 5 dpf in siblings and csf1rb mutants; red dashed circle indicates the position of the thymus. (O) Quantification of the rag1+ thymus area in siblings (n=59) and csf1rb mutant (n=19). Scale bars: 50 μm. Data are presented as mean±s.e.m. *P<0.05, **P<0.01, ****P<0.0001; ns, not significant. |
|
Csf1rb deficiency impairs the proliferation capacity of HSPCs. (A) Immunostaining of cmyb-GFP (green) and BrdU (red) in the CHT at 3 dpf in siblings and csf1rb mutants after BrdU labeling for 1 h. Yellow arrows show BrdU+ HSPCs, white arrows indicate GFP single-positive HSPCs. Scale bars: 20 μm. (B) Quantification of cmyb-GFP+ HSPCs in the CHT at 3 dpf in siblings (n=16) and csf1rb mutants (n=19). (C) Quantification of the proliferation ratio of cmyb-GFP+ HSPCs in the CHT at 3 dpf in siblings (n=16) and csf1rb mutants (n=19). Data are presented as mean±s.e.m. **P<0.01. EXPRESSION / LABELING:
PHENOTYPE:
|
|
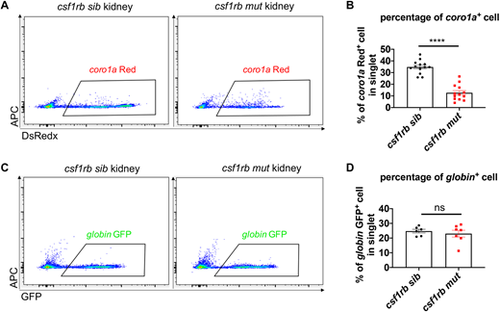
Defective leukocytes/progenitors but not erythrocytes in csf1rb mutant at adulthood. (A) Flow cytometry analysis of coro1a-DsRedx+ leukocytes/progenitors in the WKM of adult siblings and csf1rb mutants. (B) Percentage of coro1a+ cells in singlets of the WKM in siblings (n=13) and csf1rb mutants (n=13). (C) Flow cytometry analysis of globin-GFP+ erythrocytes in the WKM of adult siblings and csf1rb mutants. (D) Percentage of globin+ cells in singlets of the WKM in siblings (n=6) and csf1rb mutants (n=7). Data are presented as mean±s.e.m. ****P<0.0001; ns, not significant. |
|
Impaired engraftment ability of hematopoietic cells from adult WKM of csf1rb-deficient fish. (A) Work flow of WKM transplantation. Single-cell-stage embryos from the runx1w84x mutant were injected with pu.1 MO to create immunodeficient fish (runx1w84x; pu.1 morphant). The WKM cell suspension was prepared from csf1rb siblings and mutants with the Tg(coro1a:DsRedx) and Tg(globin:GFP) background and was transplanted into the vessel around the yolk of 2 dpf immunodeficient fish. These fish were raised to adults, and donor-derived blood cells were checked in the kidney, skin and circulation. Red circles indicate the coro1a-Red+ cells, green circles indicate the globin-GFP+ cells, the orange bar indicates the kidney, the black bar indicates the skin and the blue bar indicates the tail circulation. (B) Coro1a-Red+ cells on the skin of 3-month-old immunodeficient recipient fish after transplantation. Four out of 13 recipient fishes transplanted with WKM of sibling donor fishes were reconstituted by donor coro1a-Red+ cells on the skin (white arrows), but none of the 24 recipient fishes transplanted with WKM from csf1rb mutant donor fish had coro1a-Red+ cells on the skin. Scale bars: 200 μm. (C) Globin-GFP+ cells in the circulation in tails of 3-month-old immunodeficient recipient fish after transplantation. The four fish transplanted with WKM of sibling donors with coro1a-Red+ cells on the skin were also reconstituted by donor-derived red blood cells in the circulation (white arrows), and none of the fishes transplanted with WKM from csf1rb mutant donors had donor-derived globin-GFP+ cells in the circulation. Scale bars: 200 μm. (D) Flow cytometry analysis of WKM of 3-month-old immunodeficient recipient fish after transplantation. The four fish transplanted with WKM of sibling donors with coro1a Red+ cells on the skin were also reconstituted by donor-derived coro1a-Red+ cells in the kidney marrow, and none of the fishes transplanted with WKM of csf1rb mutant donors had donor-derived coro1a-Red+ cells in the kidney. (E) Percentage of coro1a+ cells in the WKM of recipients transplanted with the WKM cells from csf1rb siblings (n=13) or mutants (n=24). Data are presented as mean±s.e.m. |
|
Canonical ligands of Csf1r, including Csf1 and Il34, are neither required nor sufficient for HSPC expansion. (A) WISH of cmyb at 3 dpf in siblings and csf1a, csf1b and il34 single, double and triple mutants. Scale bars: 50 μm. (B) Quantification of cmyb+ HSPCs in CHT at 3 dpf in siblings and csf1a, csf1b and il34 single, double and triple mutants. n=29 (siblings); 23 (csf1amut); 22 (csf1bmut); 4 (il34mut); 36 (csf1amutcsf1bmut); 8 (csf1amutil34mut); 8 (csf1bmutil34mut); 11 (csf1amutcsf1bmut il34mut). (C) WISH of cmyb at 4 dpf in ABSR (wild type) (n=9) and Tg(hsp70:csf1a) fish (n=52). Both embryos were heat shocked at 37°C in a water bath for 1 h at 2 dpf, 2.5 dpf and 3 dpf. Red arrowheads indicate cmyb+ HSPCs. (D) WISH of cmyb at 4 dpf in ABSR (n=8) and Tg(hsp70:csf1b) fish (n=50). Both embryos were heat shocked at 37°C in a water bath for 1 h at 2 dpf, 2.5 dpf and 3 dpf. Red arrowheads indicate cmyb+ HSPCs. (E) WISH of cmyb at 3.5 dpf in ABSR (n=15) and Tg(hsp70:il34) fish (n=27). Both embryos were heat shocked at 37°C in a water bath for 1 h at 2 dpf, 2.5 dpf and 3 dpf. Red arrowheads indicate cmyb+ HSPCs. Data are presented as mean±s.e.m. ns, not significant. EXPRESSION / LABELING:
|