- Title
-
Nucleolin loss of function leads to aberrant Fibroblast Growth Factor signaling and craniofacial anomalies
- Authors
- Dash, S., Trainor, P.A.
- Source
- Full text @ Development
|
Ncl expression during zebrafish development. (A) During embryogenesis, Nucleolin (Ncl, red) was ubiquitously expressed in the cytoplasm of four-cell stage wild-type embryo at 1.5 hpf as observed by immunostaining. (B) Similarly, 3 hpf embryos also had ubiquitous cytoplasmic expression of Nucleolin. (C,C′) At 12 hpf, ncl+/+ and ncl−/− embryos exhibited similar Nucleolin expression in the nucleus and cytoplasm in most cells of the embryos. (D-D″) At 18 hpf, the expression of Nucleolin in ncl+/+ embryos was ubiquitous and was confined to the nucleus (D″). In the ncl−/− embryos, the expression pattern of Nucleolin was similar to that of wild-type embryos; however, the expression levels were significantly lower than that of the wild type. (E,E′) By 24 hpf, the expression of Nucleolin was still ubiquitous, with higher levels in the eye and the midbrain-hindbrain boundary in ncl+/+ embryos, whereas it was absent in ncl−/− embryos. (F) At 36 hpf, the expression of Nucleolin became specific to the craniofacial region in the pharyngeal arches as well as the eye. (G) In 72 hpf (3 dpf) wild-type zebrafish, Nucleolin was highly expressed in the jaw of the embryo. (F′,G′) In the ncl−/− mutants, there was no expression of Nucleolin. n=15 for each panel. The experiment was performed three times. NT, neural tube. Scale bars: 35 µm (A,B); 70 µm (C,C′); 140 µm (D,D′); 50 µm (D″); 250 µm (E,E′); 300 µm (F,F′,G,G′). EXPRESSION / LABELING:
PHENOTYPE:
|
|
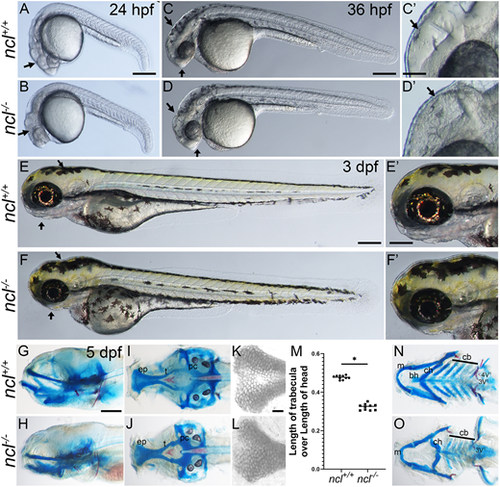
ncl−/− mutants exhibit craniofacial defects. (A,B) Compared with 24 hpf ncl+/+ clutch mates, ncl−/− mutants had necrotic tissue (indicated by black arrows) in the craniofacial region (n=25). (C,D) By 36 hpf, the frontonasal prominence and midbrain-hindbrain boundary were misshapen in ncl−/− mutants compared with their ncl+/+ siblings (indicated by black arrows) (n=50). The craniofacial region is magnified in C′,D′. (E,F) At 3 dpf, the ncl−/− mutants had smaller jaws and a misshapen head (indicated by black arrows) (n=15). The craniofacial region is magnified in E′,F′. (G,H) Skeletal preparations of 5 dpf wild-type and ncl−/− mutant zebrafish reveal defects in the cartilages of the jaw (n=50). (I,J) In the neurocranium, the chondrocytes in the ethmoid plate were delayed in development, and the trabeculae were smaller and wider compared with the wild-type zebrafish at the same stage. (K,L) Magnified images of the ethmoid plate showing differential Alcian Blue staining, as well as the loss of medial cells in ncl−/− larvae. (M) Quantification of the length of trabecula in ncl+/+ and ncl−/− embryos as a ratio of the length of the head measured from the anterior-most point of the ethmoid plate to the posterior-most point of the parachordal (pc) (n=10). Horizontal lines represent the mean. *P<0.05 (two-tailed, paired Student's t-test). (N,O) In the viscerocranium, Meckel's cartilage was bent, the basihyal was missing, the polarity of the ceratohyal was inverted and the ceratobranchials were hypoplastic. In addition, the mutants had hypoplastic teeth and the 4V1 teeth were missing. The experiment was performed three times. ep, ethmoid plate; t, trabecula; pc, parachordal; m, Meckel's cartilage; bh, basihyal; ch, ceratohyal; cb, ceratobranchial. Scale bars: 200 µm (A-F); 50 µm (C′-F′); 70 µm (G-J,N,O); 25 µm (K,L). |
|
Nucleolin is required for rRNA transcription and p53 regulation. (A) qPCR for 5′ETS, ITS1, ITS2 and 18S segment of the pre-rRNA in ncl+/+ and ncl−/− zebrafish (n=10 per sample) indicates that rRNA transcripts were significantly lower in ncl−/− embryos compared with their ncl+/+ siblings. canx was used as an internal control. (B) RNA immunoprecipitation (RIP) using a Nucleolin-specific antibody indicates that Nucleolin binds to the 5′ETS and ITS1 region of the 47S rRNA but not to the ITS2 or 18S in wild-type zebrafish (n=100 per replicate and condition). The y-axis indicates fold change of RNA pulldown compared with its absolute expression in the embryos. (C) p53 transcript expression was not significantly altered in ncl−/− mutant zebrafish between 18 and 36 hpf; however, expression of its downstream target p21 was significantly higher between 24 and 30 hpf in the ncl−/− mutants compared with wild-type zebrafish (n=5 per sample). (D) Nucleolin and IgG binding to p53 mRNA was similar in wild-type zebrafish, as observed by RNA immunoprecipitation (n=100 per replicate and condition). The y-axis indicates fold change of RNA pulldown compared with its absolute expression in the embryos. (E) p53 protein levels were higher in ncl−/− mutants at 24 hpf compared with their ncl+/+ siblings and comparable between ncl+/+ and ncl−/− embryos at 36 hpf as observed by western blotting (n=5 per sample). γ-tubulin was used as a loading control. (F) Immunoprecipitation (IP) with a Nucleolin-specific antibody followed by western blotting for p53 and Nucleolin indicates that p53 and Nucleolin bind to each other in wild-type zebrafish (n=25 per replicate and condition). (G) In ncl−/− mutants, Nucleolin expression was significantly reduced compared with controls (n=25 per replicate). α-tubulin was used as a loading control. (H) At 28 hpf, control zebrafish displayed higher binding of Mdm2 and p53 compared with that seen in mutant zebrafish (n=25 per replicate and condition). (I) Quantification of p53 protein levels in 24 hpf and 36 hpf ncl+/+ and ncl−/− embryos (n=3). (J) Quantification of p53-Mdm2 binding in ncl+/+ and ncl−/− embryos (n=3). (K-L′) ncl−/− mutants have more TUNEL+ cells (red in K,L; white in K′,L′) at 24 hpf compared with their ncl+/+ siblings (n=15 per genotype). (M-N′) By 36 hpf, apoptosis (red in M,N; white in M′,N′) is confined to the midbrain-hindbrain boundary in both ncl+/+ and ncl−/− embryos (n=15 per genotype). (O-P′) On a p53−/− mutant background, the number of TUNEL+ cells (red in O,P; white in O′,P′) in both ncl+/+ and ncl−/− embryos (n=15 per genotype) at 24 hpf is reduced. All experiments were performed three times. Scale bars: 70 µm. Data are represented as mean±s.d. in A-D; circles and squares represent individual data points and horizontal lines represent the mean in I,J. ns, not significant; *P<0.05 (two-tailed, paired Student's t-test). EXPRESSION / LABELING:
|
|
Chondrogenesis and osteogenesis defects in ncl−/− embryos. (A) qPCR revealed a significant downregulation of sox9a and col2a1 chondrogenesis markers in 36 hpf ncl−/− embryos compared with ncl+/+ embryos (n=10 per replicate). actb was used as a housekeeping control. (B,B′) Sox9a protein expression was significantly reduced in branchial arches 2-5 (white arrows) in ncl−/− embryos at 36 hpf (n=15). (C) qPCR of osteogenesis markers in 36 hpf ncl+/+ and ncl−/− embryos indicates significant upregulation in runx2a transcripts and downregulation in both col1a2 and col10a1 transcripts in ncl−/− embryos (n=10 per replicate). (D,D′) runx2a mRNA expression was significantly increased ncl−/− embryos at 3 dpf (n=15). Black arrows indicate expression of runx2a in the ceratohyal and otic vesicles. (E,E′) At 3 dpf, Runx2 protein expression was significantly increased in the palatoquadrate and the parasphenoid in ncl−/− embryos. (F) qPCR indicates a significant upregulation of the early osteoblast markers bglap and spp1 and downregulation of the late osteoblast marker sp7 in 36 hpf ncl−/− embryos compared with controls (n=10 per replicate). (G-H′) Alkaline phosphatase staining of ncl+/+ and ncl−/− embryos reveals reduced staining in the lower jaw (ventral view, black arrows) at 3 dpf (G,G′) and 5 dpf (H,H′) (n=15). All experiments were performed three times. cb, ceratobranchial. Scale bars: 200 µm (B,B′); 100 µm (D-E′,G,G′); 140 µm (H,H′). Data are represented as mean±s.d. ns, not significant; *P<0.05 (two-tailed, paired Student's t-test). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Nucleolin regulates Fgf8a expression. (A) Immunostaining of 36 hpf ncl+/+ and ncl−/− embryos with an Fgf8a-specific antibody reveals reduced expression of Fgf8a in ncl−/− embryos (n=15). (B) qPCR using craniofacial tissues from ncl+/+ and ncl−/− embryos at 18, 24, 30 and 36 hpf indicates that ncl and fgf8a expression gradually reduce over time (n=10 per replicate). (C) RNA immunoprecipitation followed by qPCR indicates higher binding of fgf8a mRNA to Nucleolin compared with the IgG control. actb was used as a negative control (n=100 per replicate per condition). The y-axis indicates fold change of RNA pulldown compared with its absolute expression in the embryos. (D-F) Skeletal preparations of 5 dpf ncl+/+ and ncl−/− larvae as controls (D) for 0.25 µg/µl (E) and 1 µg/µl (F) FGF8 exogenous treatment. Exogenous FGF8 rescued the cranioskeletal phenotype of ncl−/− larvae (n=45). Black arrows indicate the improvement of the basihyal phenotype in FGF8-treated larvae. All experiments were performed three times. Scale bars: 100 µm (A); 70 µm (D-F). Data are represented as mean±s.d. ns, not significant; *P<0.05 (two-tailed, paired Student's t-test). EXPRESSION / LABELING:
|
|
FGF8 rescues rRNA transcription in ncl−/− embryos. (A) qPCR for 5′ETS, ITS1, ITS2 and 18S in untreated and FGF8-treated ncl+/+ and ncl−/− zebrafish (n=10 per sample) indicates rescue of pre-RNA transcription in FGF8-treated ncl−/− zebrafish at 36 hpf. (B) TUNEL staining of untreated and FGF8-treated ncl+/+ and ncl−/− zebrafish at 28 hpf indicates reduced TUNEL+ cells in FGF8-treated ncl−/− embryos compared with untreated ncl−/− embryos (n=15). (C) qPCR for bmp2 in 36 hpf ncl+/+ and ncl−/− embryos (n=10 per sample) that were untreated or treated with 1 µg/µl FGF8 indicates significant downregulation in bmp2 in untreated ncl−/− embryos and significant upregulation in FGF8-treated ncl+/+ embryos. In FGF8-treated ncl−/− embryos, the bmp2 transcript levels were rescued and comparable with untreated ncl+/+ embryos. actb was used as a housekeeping control. (D) Skeletal preparations of 5 dpf ncl+/+ and ncl−/− larvae that were untreated or treated with FGF8, BMH21, FGF8+BMH21 or BMP2. Exogenous FGF8 and BMP2 treatment rescued the cranioskeletal phenotype of ncl−/− larvae (n=45). All experiments were performed three times. Scale bars: 100 µm (B); 70 µm (D). Data are represented as mean±s.d. *P<0.05 (two-tailed, paired Student's t-test). |
|
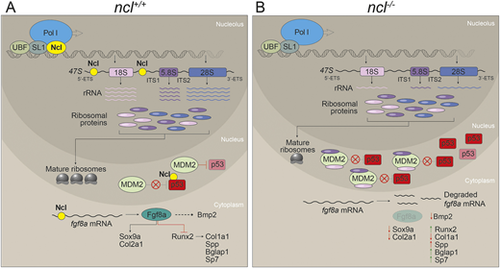
Nucleolin regulates rRNA transcription and fgf8a mRNA stability. (A) In wild-type zebrafish embryos, Nucleolin is required for rRNA transcription as well as processing. The rRNA transcripts are assembled together with ribosomal proteins to make ribosomes. Meanwhile, Mdm2 binds to and ubiquitylates p53, which results in p53 proteasomal degradation. Nucleolin binds to and stabilizes the p53 protein, thereby acting antagonistically to Mdm2. Furthermore, Nucleolin binds to and stabilizes fgf8a, which results in Fgf8a- and Bmp2-regulated chondrogenesis and osteogenesis, leading to proper craniofacial development. (B) In ncl−/− embryos, the absence of Nucleolin results in reduced rRNA transcription and possibly a free pool of ribosomal proteins that bind to Mdm2. This limits Mdm2 binding to p53, resulting in a temporary increase in p53. However, owing to the lack of Nucleolin in the cell, the p53 protein and fgf8a mRNA have reduced half-lives. This results in misregulated chondrogenesis and osteogenesis, leading to cranioskeletal defects. |