- Title
-
Therapeutic Potential of CKD-504, a Novel Selective Histone Deacetylase 6 Inhibitor, in a Zebrafish Model of Neuromuscular Junction Disorders
- Authors
- Jeong, H.S., Kim, H.J., Kim, D.H., Chung, K.W., Choi, B.O., Lee, J.E.
- Source
- Full text @ Mol. Cells
|
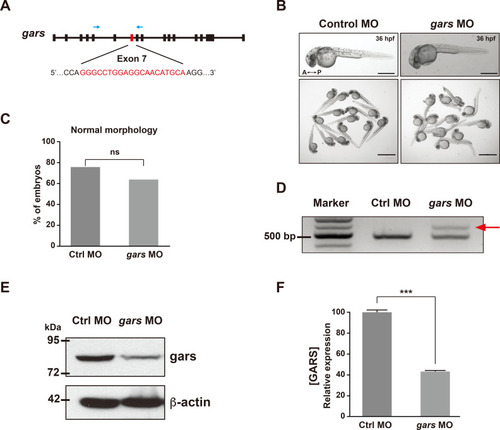
Generation of the gars-KD zebrafish model.
(A) Schematic of the target of the designed splicing-blocking morpholino oligos (MOs) on exon 7, targeting the zebrafish gars gene (red letters). Blue arrows indicate the targets of the primers used for RT-PCR showin in (D). (B) Images showing morphologies of control MOs- or gars MOs-injected larvae at 36 hpf. A, anterior; P, posterior. Scale bars = 500 μm (individual images); 2,000 μm (group images). (C) Quantification of zebrafish embryos showing morphological phenotypes, counted in each genotype at 5 dpf. Statistical significance was determined using unpaired Student’s t-test. ns, non-significant. (D) RT-PCR results confirming gars splicing on exon 7. A red arrow indicates an alternative splicing form induced by gars MOs. (E and F) Comparison of zebrafish gars expression between control and gars KD zebrafish. Results of immunoblot assays for gars and β-actin (E). Protein levels were normalized against β-actin in the same blots, and the quantification of gars expression is presented graphically in (F). Statistical significance was determined using unpaired Student’s t-test. ***P < 0.001. EXPRESSION / LABELING:
|
|
Depletion of gars results in anomalies in the NMJ in zebrafish larvae.
(A) Schematic showing the representative region where NMJs in the larval trunks were analyzed and observed. A, anterior; P, posterior; HMS, horizontal myoseptum; MS, myosepta; D, dorsal; V, ventral. Scale bars = 1,000 μm (top images) and 50 μm (bottom images). (B) Lateral view images after staining with anti-SV2 (presynaptic region) and α-BTX (postsynaptic region) of the whole-mounted larva injected with control or gars MOs at 84 hpf. Merged images (NMJ) are magnified from the images in the rectangular regions. Scale bars = 50 μm. (C and D) Comparison of fluorescence intensity (a.u.) (green, presynapse; red, postsynapse) between control MOs- (C) and gars MOs-injected (D) zebrafish embryos. (E-G) Comparisons of presynapse (E), postsynapse (F), and NMJ (G) signal ratios within the region of interest (ROI) between control MOs- (n = 21) and gars MOs-injected (n = 20) zebrafish embryos. Statistical significance was determined using unpaired Student’s t-test. *P < 0.05; ***P < 0.001. (H and I) Comparisons of NMJ innervation in the presynaptic area (H) and postsynaptic (I) areas between control MOs- (n = 15) and gars MOs-injected (n = 19) zebrafish embryos. Statistical significance was determined using unpaired Student’s t-test. ***P < 0.001. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Overexpression of human GARS restores NMJ defects in zebrafish.
(A) Lateral view images after staining with anti-SV2 and α-BTX of the whole-mounted zebrafish injected with control, gars MOs, and human WT mRNA at 84 hpf. The merged images (NMJs) are magnified from the images in the rectangular regions. Scale bars = 50 μm. (B) Comparisons of fluorescence intensity (green, presynapse; red, postsynapse) among MOs and mRNAs injected zebrafish embryos. (C-E) Comparison of presynapse (C), postsynapse (D), and NMJ (E) signal ratios within the ROI among zebrafish embryos injected with control MOs (n = 22), gars MOs (n = 22), and gars MOs with WT (n = 33) mRNAs. Statistical significance was assessed using one-way ANOVA followed by Tukey’s post hoc test. ***P < 0.001. ns, non-significant. (F and G) Comparison of NMJ innervation in the presynaptic area (F) and postsynaptic area (G) among zebrafish embryos injected with control MOs (n = 15), gars MOs (n = 19), and gars MOs with human WT GARS mRNAs (n = 30). Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test. ***P < 0.001. ns, non-significant. EXPRESSION / LABELING:
PHENOTYPE:
|
|
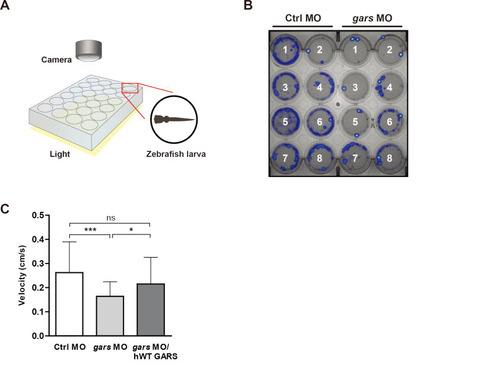
gars-KD zebrafish larvae have low motility.
(A) Simplified diagram showing how to use the DanioVision system to analyze zebrafish behaviors that occur when they respond to light stimulation. (B) Heatmap images comparing swimming patterns between control and gars MOs-injected larvae recorded for 30 min. (C) Quantification of velocity measured in zebrafish larvae injected with control MOs (n = 57), gars MOs (n = 47), and gars MOs with human WT GARS (n = 56) mRNAs. Statistical significance was assessed using one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05; ***P < 0.001. ns, non-significant. PHENOTYPE:
|
|
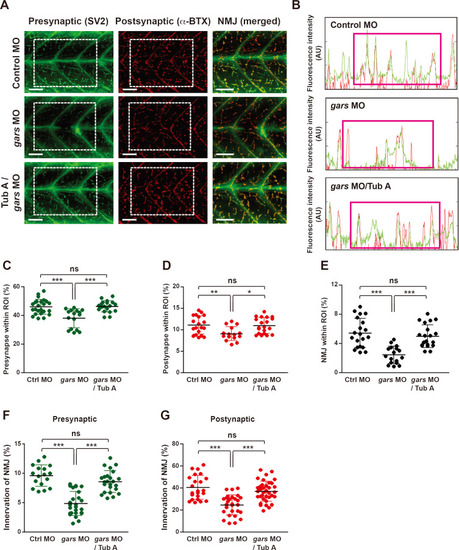
Impaired NMJs can be restored by inhibiting HDAC6.
(A) Lateral view images after staining with anti-SV2 and α-BTX of a whole-mounted zebrafish injected with control, gars MOs, or treated with tubastatin A (TubA). The merged images are magnified from the images in the rectangular regions. Scale bars = 50 μm. (B) Comparison of fluorescence intensity (green, presynapse; red, postsynapse) among MO-injected and TubA-treated zebrafish embryos. (C-E) Comparison of presynapse (C), postsynapse (D), and NMJ (E) signal ratios within the ROI among zebrafish embryos injected with control MOs (n = 24), gars MOs (n = 17), and gars MOs with TubA (n = 21). Statistical significance was assessed using one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001. ns, non-significant. (F and G) Comparison of NMJ innervation in the presynaptic (F) and postsynaptic (G) areas among zebrafish embryos injected with control MOs (n = 23), gars MOs (n = 29), and gars MOs with a TubA treatment (n = 36). Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test. ***P < 0.001. ns, non-significant. EXPRESSION / LABELING:
PHENOTYPE:
|
|
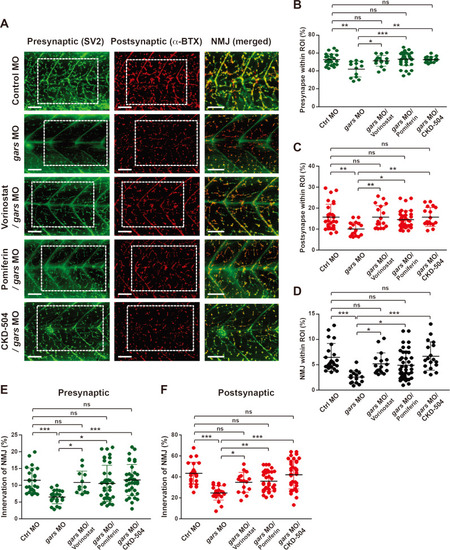
CKD-504 is an efficient HDAC6 inhibitor that repairs NMJ defects.
(A) Lateral view images after staining with anti-SV2 and α-BTX of a whole-mounted zebrafish injected with control or gars MOs, or treated with CKD-504 or FDA-approved drugs. The merged images are magnified from the images in the rectangular regions. Scale bars = 50 μm. (B-D) Comparisons of presynapse (B), postsynapse (C), and NMJ (D) signal ratios within the ROI among zebrafish embryos injected with control MOs (n = 29), gars MOs (n = 17), gars MOs with vorinostat (n = 18), pomiferin (n = 41), or CKD-504 (n = 18). Statistical significance was assessed using one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001. ns, non-significant. (E and F) Comparison of NMJ innervation in the presynaptic area (E) and postsynaptic (F) areas among zebrafish embryos injected with control MOs (n = 24); gars MOs (n = 22); and gars MOs with vorinostat (n = 16), pomiferin (n = 36), and CKD-504 (n = 36). Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001. ns, non-significant. EXPRESSION / LABELING:
PHENOTYPE:
|