- Title
-
Proper migration of lymphatic endothelial cells requires survival and guidance cues from arterial mural cells
- Authors
- Peng, D., Ando, K., Hußmann, M., Gloger, M., Skoczylas, R., Mochizuki, N., Betsholtz, C., Fukuhara, S., Schulte-Merker, S., Lawson, N.D., Koltowska, K.
- Source
- Full text @ Elife
|
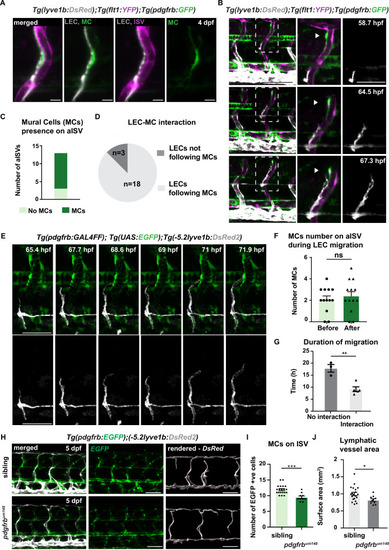
(A) Confocal stack image of trunk aISV in 4 dpf Tg(flt1:YFP); Tg(-5.2lyve1b:DsRed2);TgBAC(pdgfrb:GFP) of lymphatic endothelial cells (grey, LEC), arterial intersegments vessels (magenta, aISV) and mural cells (green, MC). Scale bar; 10 μm. (B) Confocal stack images from time-lapse images in the trunk of 2 dpf Tg(flt1:YFP); Tg(-5.2lyve1b:DsRed2); TgBAC(pdgfrb:GFP) embryos (LECs in grey). Boxed regions are enlarged (right panels). Arrowheads indicate pdgfrb+ MCs (green) next to aISVs (magenta during LEC migration). Scale bars; 100 μm or 50 μm (enlarged image). (C) Quantification of aISVs with (n=10) or without (n=3) MCs presence from n=7 embryos when LECs left HM for time lapse videos as in (E). (D) Quantification of LEC and MC interaction during migration (n=10 embryos, with four somites counted per embryo). Migrating following MC n=18, migrating not following MC n=3 from time lapse videos in (E). (E) Confocal stack images from time lapse movies of LEC migration. TgBAC(pdgfrb:GAL4FF);(UAS:GFP) in green and Tg(-5.2lyve1b:DsRed2) in grey. Scale bar: 50 μm (F) Quantification of MC number around aISVs (n=14) from n=7 embryos at the start and end of the migration, quantified from time lapse videos in (E). Data are presented as mean ± SEM, unpaired two-tailed Student’s t-test was used. Ns, no significance. (G) Quantification of duration of LEC migration with (n=5) or without (n=3) contacting MCs from n=6 embryos. Data are presented as mean ± SEM. unpaired two-tailed Student’s t-test was used. **p<0.005 (H) Confocal stack images of Tg(pdgfrb:GAL4FF); Tg(UAS:GFP) (green) and Tg(-5.2lyve1b:DsRed2) (grey) in the trunk of sibling (top) and pdgfrbum148 mutant (bottom) embryos at 5 dpf. Lymphatic vessle are rendered using lyve1b:DsRed2 channel in IMARIS s structure is rendered with lyve1b:DsRed2 channel in IMARIS (right panel). Scale bar: 100 μm. (I) Quantification of pdgfrb+ mural cell numbers around ISVs in siblings (n=20) and pdgfrbum148 mutants (n=10). Data are presented as mean ± SEM, Mann Whitney test was used. ***p<0.0001. (J) Quantification of surface area of lymphatic vasculature in siblings (n=20) and pdgfrbum148 mutants (n=10). Data are presented as mean ± SEM, unpaired two-tailed Student’s t-test was used. *p<0.05.
|
|
( |
|
( |
|
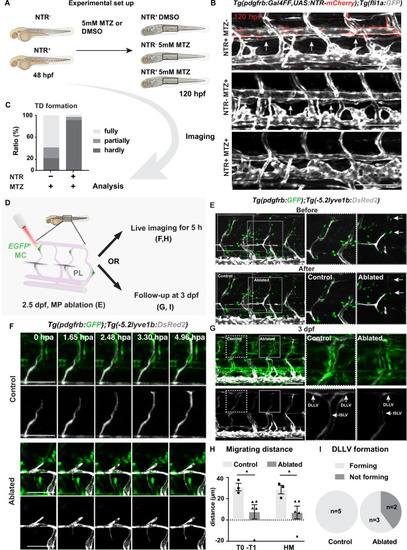
(A) Work flow of cell ablation by the nitroreductases (NTR)-metorodinazole (MTZ) system. Tg(pdgfrb:Gal4FF);Tg(14xUAS:3xFLAG-NTR,NLS-mCherry) (red) and Tg(fli1a:GFP) (grey) were imaged at 120 hours post fertilization (hpf) after treatment with DMSO or 5 mM MTZ from 48 hpf, and the formation of thoracic duct (TD) was analysed. (B) Confocal stack images of the trunk in 5 days post fertilization (dpf) embryos treated as described in (A). Arrows indicates TD forming beneath dorsal aorta. Asterisks indicate the absence of TD. Scale bar: 100 μm. (C) Quantification of (B). Embryos were scored as fully (completely connected TD), partially (partially formed TD) and hardly (almost or no TD visible) formed based on the TD development. In the NTR- MTZ+ group (n = 36), n = 21 embryos with fully formed TD, n = 7 embryos with partly formed TD, n = 8 embryos with hardly formed TD were identified. In the NTR+ MTZ+ group (n = 34), n = 1 embryo with fully formed TD, n = 2 embryos with partly formed TD, n = 31 embryos with hardly formed TD were identified. Data were presented as ratio to total number of embryos analysed. (D) Work flow of cell ablation by multi-photon microscopy. Mural cells (MCs, green) labelled by TgBAC(pdgfrb:GAL4FF; UAS:GFP) and lymphatic endothelial cells (LECs) by Tg(–5.2lyve1b:DsRed2) (grey). MCs on intersegmental vessel in proximity to sprouting LEC were ablated at 57 hpf. For analysis, ablation was either followed by time-lapse imaging or confocal imaging at 3 dpf. (E) Confocal stack images before and after ablation. Control ablation (dashed box) in the adjacent region of GFP+ MCs and GFP+ MC on arterial intersegmental vessel (aISV) (solid grey box) was performed in the same embryos. Arrows indicate ablated GFP-positive cells. Scale bar: 100 μm. Middle and right panels, zoom-in images cropped in z-stacks. (F) Live imaging of lymphatic endothelial cell migration in the context of control (top images) and GFP+ MC on aISV (bottom images) after ablation, with confocal stack images from time lapse at selected timepoints from 0 to 4.96 hpa. Scale bar: 50 μm. (G) Confocal stack images of 3 dpf embryos in (E). Dashed box, control ablation. Solid grey box, MC ablation. DLLV, dorsal longitudinal lymphatic vessel; ISLV, intersegmental lymphatic vessel. Scale bar: 100 μm. (H) Quantification of migration distance from time-lapse videos corresponding to (F). Distance was calculated as both T0-T1 and the perpendicular distance between the T1 and HM for embryos with (n = 4) or without (control, n = 3) ablation. T0, the sprouting front of LECs at the start of video; T1, sprouting front of LECs at the end of video. Data are presented as mean ± SEM, unpaired two-tailed Student’s t-test or Mann-Whitney test was used on two types of measurements respectively. *p < 0.05. (I) Quantification of DLLV formation at 3 dpf. DLLV forming (n = 3), not forming (n = 2) in the ablated group and DLLV forming (n = 5) in the control group.
|
|
( |
|
(A) Confocal stack images with transmitted light channel as in Figure 2F. Black arrows indicate erythrocytes in the blood flow, white arrowheads indicate the ablating sites. Scale bar: 50 μm. |
|
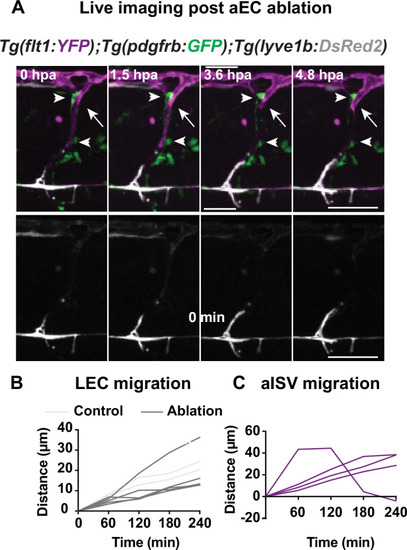
(A) Confocal stack images from time-lapse post multi-photon laser ablation in 2 days post fertilization (dpf) Tg(flt1:YFP) (magenta); TgBAC(pdgfrb:GFP) (green) and Tg(–5.2lyve1b:DsRed2) (grey) embryos. Arrowheads indicate remained GFP+ MCs without arterial intersegmental vessel (aISV). White arrows indicate the ablated site of aISV. Scale bar: 50 μm (Figure 2—figure supplement 2); arterial endothelial cell (aEC) ablation with multi-photon laser. (A–B) Quantification of lymphatic endothelial cell (LEC) (n = 4) and aISV (n = 4) migration distance post two-photon laser ablation. |
|
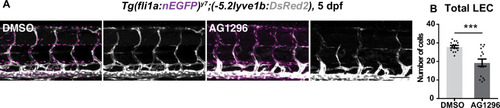
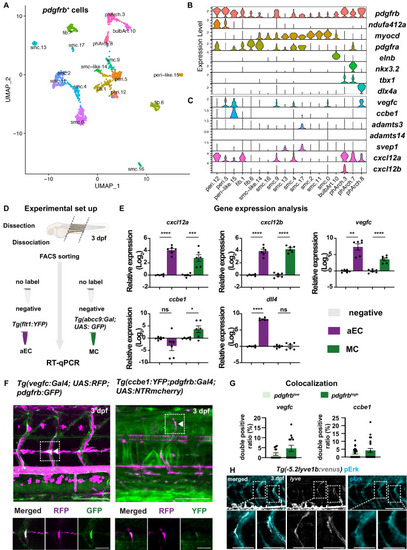
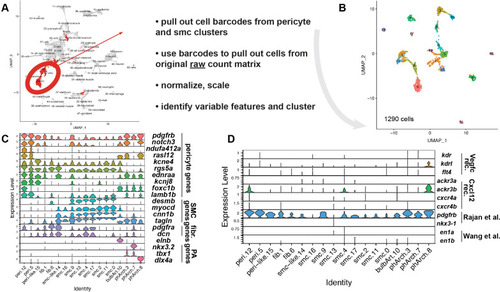
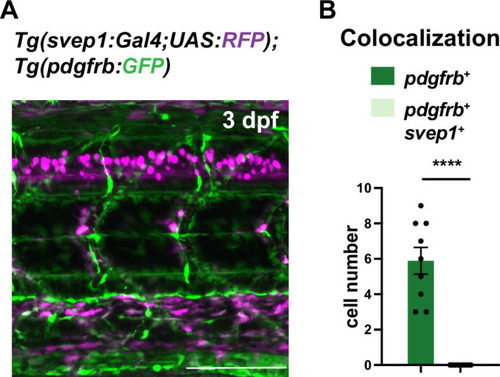
(A) Uniform Manifold Approximation and Projection (UMAP) plot of smooth muscle cells and pericytes subclustered from 5 days post fertilization (dpf) pdgfrb:egfp-positive cells. bulbArt – bulbous arteriosus, fib – fibroblast, peri – pericyte, peri-like – pericyte-like, phArch – pharyngeal arch mesenchymal cell, smc – smooth muscle cell. (B) Violin plot showing markers for pericytes (pdgfrb, ndufa4l2a), smooth muscle (myocd), fibroblasts (pdgfra), bulbous arteriosus (elnb), and pharyngeal arch mesenchyme (nkx3.2, tbx1, dlx4a). (C) Violin plot showing expression of known non-autonomous pro-lymphatic factors. Expression level values are log2 normalized across all cells. (D) Illustration of fluorescence activated cell sorting (FACS) and qPCR analysis on 3 dpf embryos. (E) qRT-qPCR of cxcl12a, cxcl12b, vegfc, ccbe1, and dll4 in FACS sorted trunk arterial endothelial cells (aECs) and MCs cells at 3 dpf as described in (D). Graph represents gene expression relative to geometric average of rpl13 and β-actin from three biological repeats (mean ± SEM). Unpaired two-tailed Student’s t-test or Mann-Whitney test was used. No significance (ns), p ≥ 0.5. *p < 0.05, ***p < 0.0005, ****p < 0.0001. (F) Confocal z-projections for immunohistochemistry of fluorescent proteins in trunks of Tg(vegfc:Gal4; UAS:RFP; pdgfrb:GFP) and confocal image of Tg(ccbe1:YFP;pdgfrb:Gal4; UAS:NTRmcherry) embryos at 3 dpf. Scale bar: 100 μm; 50 μm in enlarged images. (G) Left panel, quantification of colocalization of vegfc+ and pdgfrb+ cells based on immunohistochemistry in (F). Right panel, quantification of colocalization of ccbe1+ and pdgfrb+ cells based on confocal images in (F), data presented as double positive ratio (mean ± SEM). Ns, no significance. (H) Confocal z-projections for immunohistochemistry of endogenous pERK (cyan, right) in migrating lymphatic endothelial cells (LECs) in trunks of Tg(–5.2lyve1b:venus) embryos (α-GFP, grey, middle) (n = 10) at 3 dpf. Scale bar: 100 μm; 50 μm in enlarged images.
|
|
( |
|
( |
|
( |
|
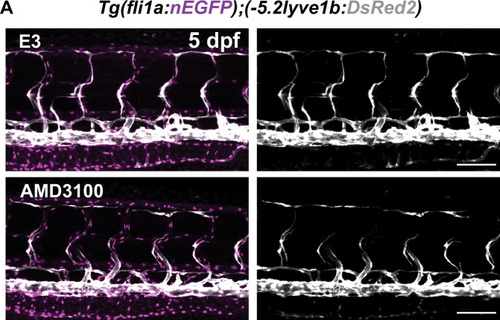
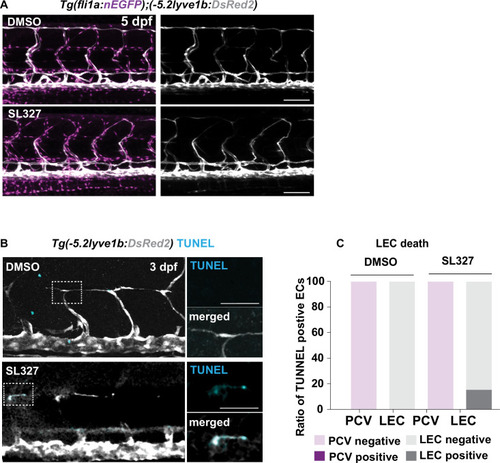
(A) Work flow of Cxcr4 inhibitor treatment. Tg(fli1:GFP);Tg(lyve1b:mCherry) embryos were grown in PTU (1-phenyl 2-thiourea) from 24 hours post fertilization (hpf) to prevent pigment formation, then changed to 20 μM AMD3100 or E3 water (embryo medium) at 51 hpf. (B) Confocal stack images from time-lapse imaging of Tg(fli1a:GFP; lyve1b:mCherry) embryos as indicated in (A). Scale bar: 50 μm. (C) (Left) Quantification of dorso-ventral migration showing individual tracks for the sprouting LECs in embryos (n = 6) in E3 water or embryos (n = 10) in AMD3100. (Right) Quantification of dorso-ventral migration showing average (mean from single tracks; left) of tracks in E3 and AMD3100-treated groups. Data are presented as mean ± SEM, unpaired two-tailed Student’s t-test was used. **p < 0.005. (D) Quantification of velocity of dorso-ventral migration from time-lapse video described in (B). Sprouting front of LECs in E3 water (n = 6) and AMD3100- (n = 10) treated embryos were tracked and the distance between starting and end position of sprouting front was measured and subsequently divided by duration. Data are presented as mean ± SEM. Unpaired two-tailed Student’s t-test was used. Ns, no significance, p > 0.1. (E) Confocal stack images from time-lapse imaging of Tg(fli1a:lifeact-EGFP);Tg(kdrl:mCherry) as indicated in (A). Arrows indicate dynamic filopodia formation during LEC migration. Scale bar: 50 μm. (F) Quantification of frequency of filopodia formation from time-lapse video from (E). Number of protrusions in LEC sprouts were counted and normalized to the sprout length, control (E3, sprouts n = 8 from 8 embryos) and treated (AMD3100, n = 12 from 10 embryos) embryos. Data are presented as mean ± SEM. Unpaired two-tailed Student’s t-test was used. ***p < 0.0005. (G) Work flow of MEK inhibitor treatment. Tg(fli1:GFP);Tg(lyve1b:mCherry) embryos were grown in PTU from 24 hpf, then changed to 10 μM SL327, a MEK inhibitor, or DMSO at 51 hpf. Time-lapse imaging was started at 57 hpf. (H) Confocal z-stack images from time lapse of 57 hpf Tg(fli1a:nEGFP)y7 (green) and Tg(–5.2lyve1b:DsRed2) (grey) embryos treated with DMSO or 10 μM SL327 from 51 hpf. Grey arrowheads indicate cell death. Scale bar: 50 μm. (I) (Left) Quantification of dorso-ventral migration showing individual cell tracks for nuclei of sprouting LECs in DMSO- (embryos, n = 10; left panel) and SL327- (embryos, n = 9; right panel) treated embryos as described in (H). Red cross indicates cell death at the end of tracking. (Right) Average (mean) of tracks in DMSO- and SL327-treated groups. Data are presented as mean ± SEM, unpaired two-tailed Student’s t-test was used. ****p < 0.0001. (J) Quantification of total LEC numbers at beginning (T0) and end (T1) of the time lapse of embryos (n = 10) in DMSO- and SL327-treated embryos (n = 9); data are presented as mean ± SEM. T0 DMSO vs. T1 SL327 p < 0.0001, T0 SL327 vs. T1 SL327 p < 0.0001, T1 DMSO vs. T1 SL327 p < 0.0001. Other comparisons were ns. One-way ANOVA with Tukey’s post hoc test for statistical analysis. ****p < 0.0001. (K) Quantification of cell proliferation in DMSO (n = 9) and SL327-treated (n = 13) embryos as described in (E). Nuclear marker in green was used to count cell division events. Data are presented as mean ± SEM, Mann-Whitney test was used. ****p < 0.0001. |
|
( |
|
( |
|
Vegfc-Vegfr together with Cxcl12-Cxcr4 coordinate lymphatic endothelial cell (LEC) migration. |
|
( |
|
(A) Endogenous pERK (cyan) in migrating lymphatic endothelial cell (LEC) in trunk of 3 dpf Tg(hsp70l;sflt4, cryaa:Cerulean) and Tg(flt1:YFP;lyve1b:DsRed2) embryos (α-DsRed2, grey) with treatment as described in Figure 5A. Box indicates enlarged area. Scale bar: 100 μm; 50 μm in enlarged images.
|