- Title
-
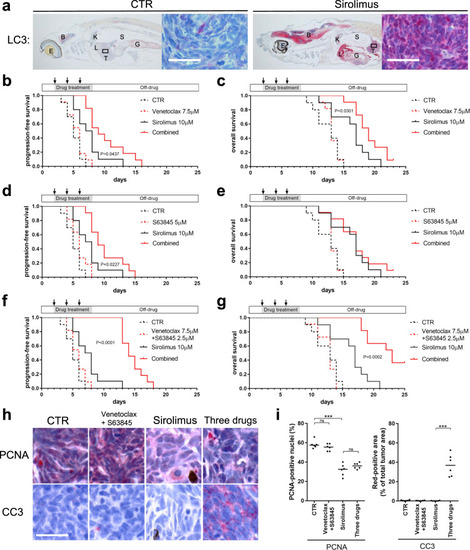
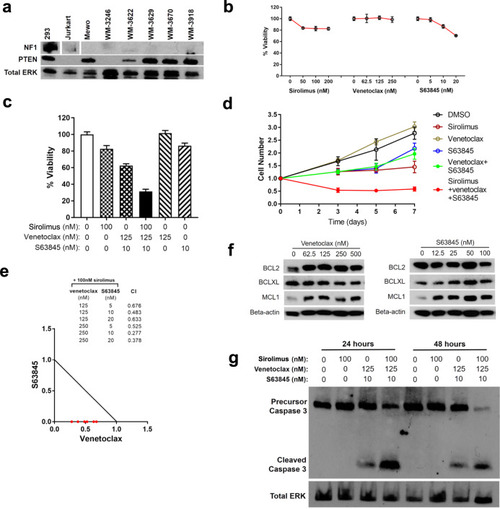
Synergistic melanoma cell death mediated by inhibition of both MCL1 and BCL2 in high-risk tumors driven by NF1/PTEN loss
- Authors
- He, S., Zimmerman, M.W., Layden, H.M., Berezovskaya, A., Etchin, J., Martel, M.W., Thurston, G., Jing, C.B., van Rooijen, E., Kaufman, C.K., Rodig, S.J., Zon, L.I., Patton, E.E., Mansour, M.R., Look, A.T.
- Source
- Full text @ Oncogene
|
|
|
|
|
Three-week-old |
|
|
|
|
|
|
|
|