- Title
-
Genetic Modeling of the Neurodegenerative Disease Spinocerebellar Ataxia Type 1 in Zebrafish
- Authors
- Elsaey, M.A., Namikawa, K., Köster, R.W.
- Source
- Full text @ Int. J. Mol. Sci.
|
Generation of a stable transgenic zebrafish model for PC-specific SCA1. (A) Schematic drawing (not to scale) of expression constructs for PC-specific expression of red fluorescent reporter protein mScarlet targeted to the cytoplasmic membrane due to an N-terminal fusion with a post translational palmitoylation signal derived from the zebrafish GAP43 protein. This reporter is expressed alone, or coexpressed with non-pathological [30Q] and mutant pathological [82Q] alleles of human ataxin1. Ectopic expression in tectal neurons and retinal cells was eliminated by four copies of miRNA181a target sites (4xmiR181aT) in the 3′UTR of the transgenes [25]. All bidirectional transgene cassettes are flanked by Tol1 transposase recognition sites [26]. (B) Schematic outline of screening and breeding procedure to obtain stable transgenic zebrafish of the F2-generation, which propagate PC-specific mScarlet and Atx1 expression in a Mendelian manner. (C) Analysis of GAPmScarlet expression (a–c) of carriers crossed into the transgenic Tg(−7.5ca8:GFP)bz12 background with PC-specific cytoplasmic EGFP expression (d–f) and merged images (g–i) to confirm PC-specific coexpression of the transgene cassette in F2 larvae at 4 dpf of the established transgenic control mScarlet (upper row), Atx1[30Q] (middle row), and Atx1[82Q] (lower row) strains, respectively. The individual images display dorsal views of the larval corpus cerebelli, anterior is to the left. Scale bar: 50 µm. EXPRESSION / LABELING:
|
|
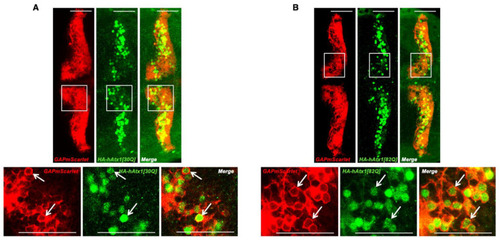
Analysis of human Atx1 allele expression in the stable transgenic zebrafish strains. Anti-HA immunostaining of HA-tagged proteins hAtx1[30Q] (A) and hAtx1[82Q] (B) in transgenic 4 dpf F2 larvae of established Atx1[30Q] and Atx1[82Q] strains crossed into the transgenic Tg(−7.5ca8:GFP)bz12 backgroundCytoplasmic and nuclear anti-HA-derived green fluorescence is surrounded by PC-specific membrane targeted red fluorescent GAPmScarlet expression (white arrows) The images display a dorsal view of the larval zebrafish corpus cerebelli, anterior is to the left. While in the upper row an overview over both cerebellar hemispheres is shown, the white boxes depict an area that was displayed at higher magnification in the lower row. Scale bars: 50 µm. EXPRESSION / LABELING:
|
|
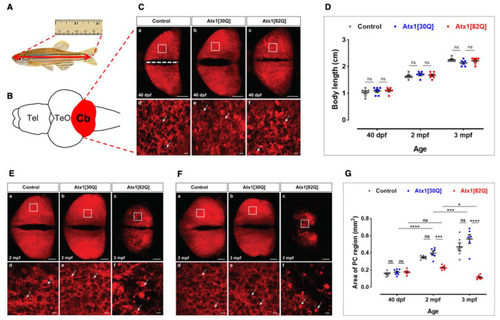
Progredient disintegration of PC layer in genetic model of SCA1 in zebrafish. (A) Schematic drawing of adult zebrafish to demonstrate measurement of body length form the anterior tip of the jaw until the caudal indentation of the tail fin. (B) The cerebellum in dissected brains from these specimens is easy to identify by the red fluorescent PC population forming a continuous layer of cells across the corpus cerebelli (dorsal view). (C) Maximum brightness projection of images stacks recorded by confocal microscopy from control (a,d), Atx1[30Q] (b,e), and Atx1[82Q] (c,f) expressing heterozygous carriers of the respective transgene at 40 dpf. Note speckles of brighter fluorescence suggestive of condensation and cellular shrinkage can be observed in the PC layer of Atx1[82Q] carriers not found in GAPmScarlet and Atx1[30Q] controls (compare white arrows). (D) Body length measurements reveal a similar overall growth rate to control (n = 6), Atx1[30Q] (n = 6), and Atx1[82Q] (n = 6) fish. (E) Maximum brightness projection of image stacks recorded by confocal microscopy from control (a,d), Atx1[30Q] (b,e), and Atx1[82Q] (c,f) expressing heterozygous carriers of the respective transgene at two months of age and (F) three months of age, respectively. The area marked by a white rectangle is displayed at higher magnification below for each specimen, respectively. Compared to controls and Atx1[30Q], young adult Atx1[82Q] zebrafish at three months of age display a clear atrophy of their PC layer containing widespread fluorescent debris of degenerated PCs (Ff white arrows). (G) Quantification of area covered by fluorescent PCs, while in controls (n = 6) and Atx1[30Q] carriers (n = 6) a continuous age-dependent increase of the PC layer occurs, the expansion of the PC layer in Atx1[82Q] carriers (n = 6) stalls. (C,E,F): dorsal views of corpus cerebelli, anterior to the left, scale bars: 100 µm (a–c) or 10 µm (d–f). The data in (D,G) are presented as mean ± SEM, * p < 0.05, *** p < 0.001 and **** p < 0.0001 according to two-way ANOVA with post hoc Tukey’s test. n = 7, 6, 6 in 40 dpf, 2 mpf (months postfertilization) and 3 mpf control groups, respectively. n = 7, 6, 6 in 40 dpf, 2 mpf, and 3 mpf Atx1[30Q] groups; and n = 6, 7, 7 in 40 dpf, 2 mpf, and 3 mpf Atx1[82Q] groups, respectively. Abbr.: Cb: cerebellum, PC: Purkinje cell, TeO: optic tectum, Tel: telencephalon. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Histological comparison of adult corpus cerebelli of control and hAtx1 expressing zebrafish. Sagittal cryogenic sections through the corpus cerebelli of three months old adult zebrafish from transgenic GAPmScarlet controls (upper row), Atx1[30Q] (middle row) and Atx1[82Q] (lower row) heterozygous carriers, anterior is to the left. (a–c) Hematoxilin and Eosin staining reveals no changes in gross morphology of the corpus cerebelli in overview images, yet at higher magnifications (d–f) large somata (white arrows) characteristic for PCs juxtaposed to the GCL are only visible in control and Atx1[30Q] cerebelli but are hardly found in Atx1[82Q] cerebelli. This absence of PCs is further supported by different patterns of red GAPmScarlet fluorescence, which in controls (g) and Atx1[30Q] (h) samples appears as a continuous layer (white arrow), but can be found in cerebellar tissue of Atx1[82Q] carriers (i) only in posterior regions which is further confirmed by green fluorescent immunohistochemistry using the PC-specific ZebrinII-antibody (j–l). Green fluorescent anti-HA-tag immunohistochemistry confirms the maintenance of human Atx1-protein expression in PCs, yet compared to Atx1[30Q] cerebelli (m) only with sparse labelling in PCs (white arrows) of Atx1[82Q] carriers (n) suggesting a progressive state of degeneration. Scale bars: 100 µm (a–c,g–l), 10 µm (d–f,m,n). Abbr.: GCL: granule cell layer, ML: molecular layer, PCL: Purkinje cell layer. |
|
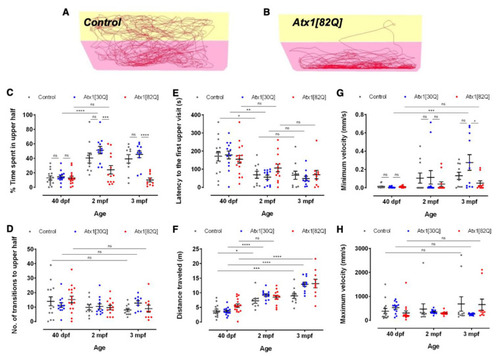
Age-dependent exploratory behavioural comparison of control and hAtx1 expressing zebrafish. Novel tank diving tests were performed with GAPmScarlet, Atx1[30Q], and Atx1[82Q] larvae at 40 dpf, juveniles at two months and young adults at three months of age, respectively. Examples of 6-min traces for (A) a GAPmScarlet control fish and (B) an Atx1[82Q] carrier are shown, the bottom half of the tank is marked in pink, the upper half in yellow. (C) Percentage of time spent in the upper half of the novel tank during a 6-min observation period. (D) Number of entries into upper tank half during the entire observation period. (E) Latency until upper tank half is entered for the first time. (F) Total distance travelled during the entire observation period. (G) Minimal and (H) maximal velocity of swim movements measured during the observation period. Data are presented as mean ± SEM, * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 (according to two-way ANOVA with post hoc Tukey’s test). n = 14,10,10 in 40 dpf, 2 mpf, and 3 mpf control groups; n = 13, 12, 11 in 40 dpf, 2 mpf and 3 mpf Atx1[30Q] groups; and n = 15, 13, 10 in 40 dpf, 2 mpf, and 3 mpf Atx1[82Q] groups, respectively. PHENOTYPE:
|