- Title
-
Loss of Gap Junction Delta-2 (GJD2) gene orthologs leads to refractive error in zebrafish
- Authors
- Quint, W.H., Tadema, K.C.D., de Vrieze, E., Lukowicz, R.M., Broekman, S., Winkelman, B.H.J., Hoevenaars, M., de Gruiter, H.M., van Wijk, E., Schaeffel, F., Meester-Smoor, M., Miller, A.C., Willemsen, R., Klaver, C.C.W., Iglesias, A.I.
- Source
- Full text @ Commun Biol
|
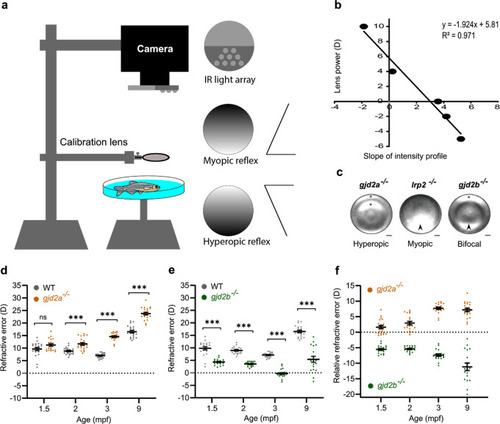
SD-OCT recordings of size-matched juvenile-to-adult zebrafish indicating the temporal dynamics of the eye. All ocular metrics were corrected for the tissue-specific refractive index. PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
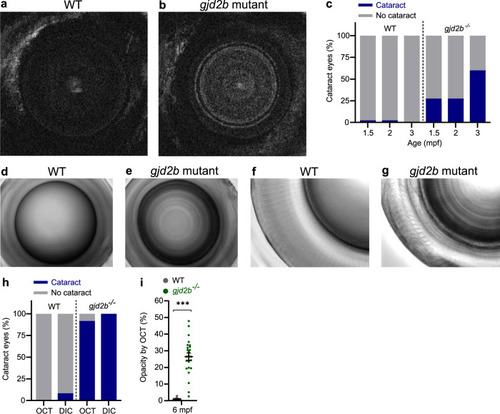
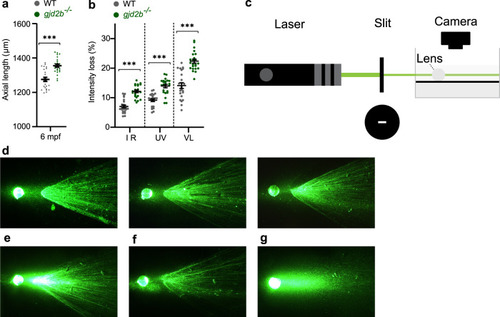
a SD-OCT shows a significant increase in axial length in 6 mpf gjd2b (Cx35.1) mutants (n = 24 eyes). b Loss of lenticular projection intensity of isolated mutant lenses (n = 24 eyes), the following wavelengths were used: IR light (940 nm), UV light (365 nm), and broad-spectrum visible light (380–760 nm). c Schematic illustration of the ex vivo lenticular light propagation setup. d Examples of light propagation in three isolated 6 mpf WT control lenses. e–g Examples of light scattering and multifocality by two moderately cataractous lenses (e) and (f) and light diffusion by a severely cataractous lens (g) of the 6 mpf gjd2b (Cx35.1) mutant. Error bars: SEM. Significance: ns = not significant, *p < 0.05, **p < 0.01, ***p < 0.001. IR infrared, UV ultraviolet. |
|
|
|
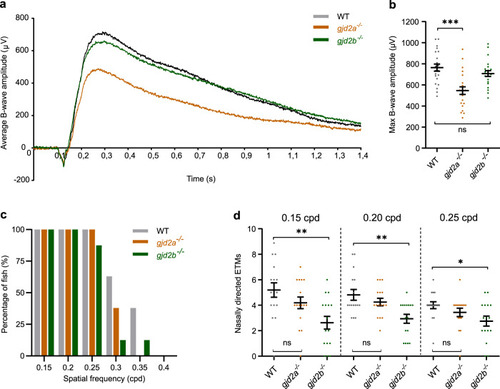
Immunostaining showing the topographic distribution of Cx35.5 ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
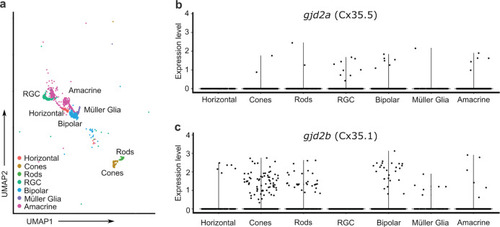
Single-cell transcriptome analysis reveals |
|
Simplified illustration summarizing the relation between (un)coupling of retinal gap junctions and axial eye growth. We hypothesize, based on reported literature and the outcome of this study (asterisk), that the (un)coupling of retinal gap junctions may play an intermediate role in controlling emmetropization. The exact mechanism of how Cx35/Cx36 uncoupling leads to reduce axial eye growth is unclear (dotted line arrow). |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|