- Title
-
Xmrks the Spot: Fish Models for Investigating Epidermal Growth Factor Receptor Signaling in Cancer Research
- Authors
- Monroe, J.D., Basheer, F., Gibert, Y.
- Source
- Full text @ Cells
|
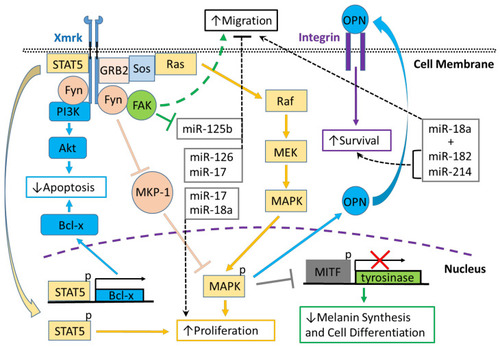
Xmrk signaling in the Xiphophorus melanoma model. Xiphophorus melanoma receptor kinase (Xmrk) is a receptor tyrosine kinase that recruits and activates several kinases, transcription factors and adaptor proteins integrated into signaling networks to regulate pigment cell cancer transformation. Activation of the kinase, phosphoinositide 3-kinase (PI3K), prevents apoptosis in malignant cells, as does activation of the transcription factor, signal transducer and activator of transcription 5 (STAT5), via B-cell lymphoma (Bcl-x) signaling, and STAT5 also promotes proliferation. The Src kinase protein, Fyn, acts as a docking protein and, through focal adhesion kinase (FAK), can induce pigment cell migration. Further, Fyn inhibits MAPK phosphatase 1 (MKP-1), a repressor of MAPK, causing the promotion of MAPK’s function to increase melanoma cell proliferation. Xmrk can also signal via the adaptor proteins, GRB2 and Sos, to activate the Ras/Raf/MEK/MAPK pathway, thereby enhancing proliferation. Additionally, MAPK signaling causes degradation of the transcription factor, microphthalmia transcription factor (MITF), leading to reduced transcription of tyrosinase, which decreases melanin synthesis and inhibits pigment cell differentiation. Another function of activated MAPK is to induce the synthesis of osteopontin (OPN), which is secreted, binds to integrins on the cell membrane surface, and promotes pigment cell survival. Several miRNAs that are regulated in fish Xmrk models also have functions in human melanoma. The miRNAs miR-18a, -182, and -214, can act to promote migration in human melanoma, while miR-182 and -214 can also stimulate cell survival. MiR-17, 125b, and -126 act to inhibit melanoma cell migration, with -125b’s action inhibited by FAK. MiR-17, along with miR-18a, also function to increase melanoma proliferation. |
|
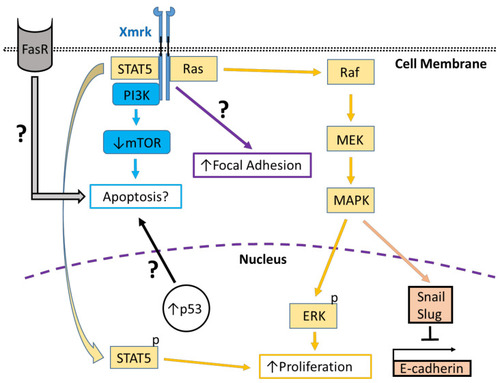
Xmrk signaling in the zebrafish hepatocellular carcinoma model. In transgenic zebrafish models of HCC, Xmrk signals through kinases and transcription factors that modulate liver cancer progression. PI3K activation may modulate apoptosis through the mechanistic target of rapamycin (mTOR), although mTOR expression may be decreased in HCC, making its effect on apoptosis uncertain. STAT5 signaling can promote proliferation, as does MAPK via activation of extracellular signal-regulated kinases (ERK). MAPK also has a dual role in signaling through the transcription factors, Snail and Slug, to inhibit E-cadherin activity. The formation of focal adhesions is increased through a pathway that is currently uncharacterized. Xmrk is also associated with increased expression of the tumor suppressor, p53, and the Fas receptor (FasR), which regulates apoptosis during HCC, but the details of their associated signaling mechanisms are not currently understood. |